Yeni ve ?nceden tedavi edilmi? ekstrapulmoner
t?berk?lozlu hastalar?n h?zl? tan?s?nda
IS6110 ile konvansiyonel y?ntemlerin
kar??la?t?r?lmal? de?erlendirilmesi
Anand Kumar MAURYA1, Surya KANT1, Vijaya Lakshmi NAG2, Ram Awadh Singh KUSHWAHA1,
Manoj KUMAR2, Tapan N. DHOLE2
1 Chhatrapati Shahu Ji Maharaj T?p ?niversitesi, G???s Hastal?klar? B?l?m?, Lucknow, Hindistan,
2 Sanjay Gandhi Lisans?st? T?p Bilimleri Enstit?s?, Mikrobiyoloji B?l?m?, Lucknow, Hindistan,
?ZET
Yeni ve ?nceden tedavi edilmi? ekstrapulmoner t?berk?lozlu hastalar?n h?zl? tan?s?nda IS6110 ile konvansiyonel y?ntemlerin kar??la?t?r?lmal? de?erlendirilmesi
Geli?mekte olan ?lkelerde ekstrapulmoner t?berk?loz (EPTB) tan?s? ?nemli bir problemdir. EPTB?de, az say?da basil i?erme ?zelli?i, yetersiz miktarda ?rnek gibi bir?ok sorun bulunmaktad?r. B?t?n bu k?s?tlamalar, konvansiyonel bakteriyolojik tekniklerin EPTB tan?s?na d???k katk?s?na neden olmaktad?r. N?kleik asit amplifikasyon y?ntemleri, mikobakteriyel DNA?n?n saptanmas? amac?yla geli?tirilen h?zl? ve duyarl? tekniklerdir. Mycobacterium tuberculosis complex?in spesifik genomunda yer alan ?insertion sequence? IS6110?a ait 123bp?nin DNA fragman?, EPTB?nin h?zl? tan?s? amac?yla polimeraz zincir reaksiyonu (PCR) ile ?o?alt?ld?. Bu ?al??mada, yeni ve ?nceden tedavi edilmi? EPTB?li hastalar?n h?zl? tan?s?nda IS6110 PCR ile konvansiyonel y?ntemler kar??la?t?r?ld?. EPTB ??pheli hastalardan 450 ?rnek topland? ve Mycobacteria i?in Zeihl Neelson (ZN) boyama ve M. tuberculosis i?in BACTEC k?lt?r? yap?ld?. B?t?n ?rnekler ayr?ca, M. tuberculosis complex?in insertion element IS6110?un 123bp fragman?n? hedefleyen primerlerle PCR amplifikasyonu ile IS6110 i?in ?al???ld?. Testler aras?nda duyarl?l?k bak?m?ndan anlaml? fark saptand?. D?rt y?z elli ?rnek i?inde, 60 (%13.4)??nda ZN boyamada ARB pozitif, 202 (%45)?sinde BACTEC k?lt?r? ve 283 (%63)??nde M. tuberculosis complex i?in IS6110 PCR pozitifti (p< 0.05). Bununla birlikte, testler aras?nda spesifisite bak?m?ndan anlaml? fark yoktu (p> 0.05). IS6110 PCR?nin hem yeni hem de ?nceden tedavi edilmi? hastalarda, yayma mikroskopi ve BACTEC k?lt?r?nden daha duyarl? oldu?unu bulduk. IS6110 PCR, yeni ve ?nceden tedavi edilmi? EPTB?li hastalar?n tan?s?nda kullan??l? olabilir. ??pheli EPTB?li hastalar?n tedavi karar?nda fayda sa?layabilir.?
Anahtar Kelimeler: T?berk?loz, ekstrapulmoner t?berk?loz, polimeraz zincir reaksiyonu, IS6110.
SUMMARY
Comparative evaluation of IS6110 PCR via conventional methods in rapid diagnosis of new and previously treated cases of extrapulmonary tuberculosis
Anand Kumar MAURYA1, Surya KANT1, Vijaya Lakshmi NAG2, Ram Awadh Singh KUSHWAHA1,
Manoj KUMAR2, Tapan N. DHOLE2
1 Department of Chest Diseases, Chhatrapati Shahu Ji Maharaj Medical University UP, Lucknow, India,
2 Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India,
In developing countries the diagnosis of extrapulmonary tuberculosis (EPTB) is a major burning challenge. EPTB encounters many problems like pauci-bacillary nature, inadequate specimen volume. All the limitations reflect in the poor contribution of conventional bacteriological technique in the establishment of diagnosis of EPTB. Nucleic acid amplification methods are rapid and sensitive has modified strategies for the detection of mycobacterial DNA. A fragment of DNA of 123bp belonging to insertion sequence IS6110 based on specific gene of Mycobacterium tuberculosis complex was amplified by polymerase chain reaction (PCR) for the rapid diagnosis of EPTB. The present study was to comparative evaluation of IS6110 PCR via conventional methods in the rapid diagnosis of new and Previously treated cases of extra pulmonary tuberculosis. Four hundred fifty specimens were collected from suspected cases of EPTB were processed for Mycobacteria by Zeihl Neelson (ZN) staining and BACTEC culture for M. tuberculosis. All the specimens were also processed for IS6110 based PCR amplification with primers targeting 123 bp fragment of insertion element IS6110 of M. tuberculosis complex. We found significant difference was seen in sensitivities of different tests. Of these 450 specimens, 60 (13.4%) were positive for AFB by ZN staining, 202 (45%) for BACTEC culture and IS6110 PCR were positive for M. tuberculosis complex in 283 (63%) specimens (p< 0.05). However, there was no significant difference (p< 0.05) as far as specificity of different tests. We found that IS6110 PCR has higher sensitivity than smear microscopy and BACTEC culture in both cases of new cases as well as in previously treated cases. IS6110 PCR can be highly useful in diagnosis of new and treated cases of EPTB. It may facilitate therapeutic decisions for those with suspected of EPTB.
Key Words: Tuberculosis, extrapulmonary tuberculosis, polymerase chain reaction, IS6110.
Tuberculosis (TB) continues to be a major global public health problem. Incidence of extrapulmonary tuberculosis (EPTB) is on increasing worldwide as well as in India (1,2). EPTB compromises 20% of all TB cases in India (3). Diagnosis of EPTB in different clinical presentations has been always as challenge. Smear microscopy and culture lack of sensitivity in EPTB case and culture (solid and liquid media) also takes at least two to four weeks for grow of mycobacteria. A study has reported smear positive is around 10-37% of the patients and mycobacterial culture is positive in variable proportional 12-80% in different biological specimens (3). Studies from many laboratories around the global were using primers most commonly targeting the IS6110 insertion element (4,5,6,7,8,9). The detection of the IS6110 insertion element present in form of multiple copies to detect of Mycobacterium tuberculosis complex but not other mycobacterial species (9,10,11). Polymerase chain reaction (PCR) using IS6110 insertion sequences as the target, has potential to conquer limitation of conventional method and to established as rapid, sensitive technique for detecting DNA of M. tuberculosis in different clinical specimens from respiratory and non respiratory sites (10,12,13). Author has provided results 61.5% sensitivity for smear negative samples up to 87.8% specificity on M. tuberculosis culture (5). Various studies were reveled that sensitivity of PCR in these ranges 42-93% (14,15,16,17). Various studies showed that PCR can be used for the detection of pulmonary TB cases in routine laboratories (18,19,20). Few studies shown data on detection of M. tuberculosis in extrapulmonary tuberculosis cases but results were not defined on basis of new and previously treated of EPTB. The aim of this study was to comparative evaluation of IS6110 PCR vs. conventional methods in the diagnosis of new and previously treated cases of extrapulmonary
MaterialS and Methods
Study Design
The study was performed prospectively in a blinded manner.
Study Population
Study setting was Referral Medical Institutions at Northern India. Indoor and Outdoor, Department of Pulmonary Medicine, Chhatrapati Shahu Ji Maharaj Medical University UP, Lucknow, India (Erstwhile King George Medical University) and Mycobacteriology Laboratory, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India.
Clinical Specimens and Data Collection
2-5 mL of specimens was collected from 450 specimens, non-repeated specimens from suspected cases of extrapulmonary tuberculosis. The specimens were included as Lymph Node Aspirate and Cold Abscesses, Pleural fluid, C.S.F, Synovial Fluid, Ascetic Fluid, Urine, Gastric Aspirate, Pus, Bone Marrow, Wound and Pus swab and Others specimens (biopsies tissues). All specimens were kept in ice box and transported Mycobacteriology Laboratory, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India for smear examination by ZN Staining, BACTEC Culture and PCR test. All patients were signed with due informed consent of the patients from indoor and outward wards of Department of Pulmonary Medicine, Chhatrapati Shahuji Maharaj Medical University, Lucknow, India and Mycobacteriology Laboratory, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India during Jan 2009 to Dec 2010. The clinical history regarding, present and past history of antitubercular treatment (ATT); family history of tuberculosis and any other associated disease were taken in prescribed Performa.
Microbiological Analysis of Extra
Pulmonary Specimens
Specimens was divided in to two part one part was kept at -20 for PCR till processing and another part was processed for mycobacterial smear preparation and BACTEC culture. Smears were stained with Ziehl Neelsen (ZN) method and examined for acid-fast bacilli (AFB) (21). BACTEC vials were incubated and interpreted as per Becton Dickinson (BD, Sparks, MD, USA) manual instructions (22). NAP (p-nitro-a-acetylamino-b-hydroxy propiophenone) (Becton Dickinson, Sparks, MD, USA), identification was done to differentiate M. tuberculosis form non tuberculous mycobacteria (22). A decrease or unchanged growth index (GI) in nap vial indicated presence of M. tuberculosis complex (MTBC), while an increase in GI indicated the presence of Mycobacterium other than tuberculosis (MOTT). Standard H37 Rv strain of M. tuberculosis complex was used as positive control.
Extraction of DNA
Extraction of DNA was done by the CTAB (cetyl-tri-methyl-ammonium bromide) -phenol chloroform extraction method (23). Specimens were centrifuged at 10.000 rpm for 10 min. The supernatant was discarded and the pellet suspended in 567 ?L of TE (Tris EDTA, pH 7.4) buffer, 30 ?L 10% SDS (sodium dodecyl sulfate) and 3 ?L proteinase K (20 mg/mL), mixed and incubated at 37?C for 1 hour. After incubation, 100 ?L of 5 M NaCl and 80 ?L of high-salt CTAB buffer (containing 4 M NaCl, 1.8% CTAB was added and mixed followed by incubation at 65?C for 10 min. An approximate equal volume (0.7-0.8 ?L) of chloroform-isoamyl alcohol (24.1) was added, mixed thoroughly and centrifuged for 4-5 min in a microcentrifuge at 12.000 rpm. The aqueous viscous supernatant was carefully decanted and transferred to a new tube. An equal volume of phenol: chloroform-isoamyl alcohol (1:1) was added followed by a 5 min spin at 12.000 rpm. The supernatant was separated and then mixed with 0.6 volume of isopropanol to get a precipitate. The precipitated nucleic acids were washed with 75% ethanol, dried and re-suspended in 100 ?L of TE buffer.
Primer and IS6110 PCR
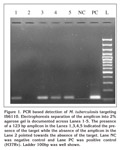
The amplification reaction was performed in a final volume of 20 ?L. the reaction mixture contained 10 ?L Pyrostart Fast PCR Master Mix 2X (dNTP, Taq polymerase with Mgcl2, Fermentas, India), 1 ?L (10 pmole) of each primer, 3 ?L water (nuclease free) and 5 ?L of extracted DNA. The oligonucleotide primers used were IS1 and IS2, are: 5?-CCT GCG AGC GTA GGC GTC GG3? and 5? CTC GTC CAG CGC CGC TTC GG 3? respectively (SBS Gentech Co. Ltd) (24). These primers amplified a target fragment at 123 base pairs (bp) from the insertion, M. tuberculosis sequence element IS6110. The PCR amplification was done in thermal cycler (MJ Research, PTC-100, GMI, Inc, USA), which involved 40 cycles of denaturation at 94?C for 2 minute, annealing of primers at 68?C for 2 minute, and primer extension at 72?C for 1 minute. The amplified products were separated on 2% agarose gels, visualized on a UV-light transilluminator (Bangalore Genei, Bangalore, India). The presence of 123bp fragment indicate as positive test as M. tuberculosis complex. The positive controls included the DNA of H37Rv strain. Negative control included PCR grade water (Figure 1).
Statistical Analysis
Data were analyzed using SPSS 15.0 (Statistical Package for the Social Sciences, Chicago, IL, USA) for Windows. The significance of difference was taken as significance value (p< 0.05).Sensitivity was calculated as [Tp/(Tp + Fn)] x 100; specificity was calculated as [Tn/(Tn + Fp)] x 100; Tp = total number of positives; Tn = total number of negatives; Fp = total number of false positive, Fn = total number of false negative; respectively.
Results
Specimen?s Characterization of
Extrapulmonary
Tuberculosis Cases
During the two year study period, 470 clinical specimens were strong clinical suspicion of extrapulmonary tuberculosis were subjected from tertiary care hospitals and all mention test were performed. Out of these, 20 specimens found to be contaminated in BACTEC culture. 450 specimens of results were used in the study. Out of 450 specimens, 153 (34%) lymph node aspirate and cold abscesses, 58 (12.8%) pleural fluid, 44 (9.7%) cerebrum spinal fluid (CSF), 48 (10.7%) urine, 31(6.8%) ascetic fluid, 26 (5.8%) pus, 22 (4.9%) wound and pus swab, 16 (3.5%) gastric aspirate, 10 (2.2%) bone marrow, 10 (2.2%) synovial fluid and 30 (6.7%) others specimens (biopsies tissues). Out of 450 patients, 320 (71.1%) patients were males and 130 (28.9%) females. The mean age of all patients was 39.8 ? 16.1 years. Patients 25-44 years of age accounted for 45% of the total cases. Out of 450 cases, 328 (72.8%) were new cases and 122 (22.2%) were previously treated cases of EPTB.
Detection Rate of M. tuberculosis by
IS6110 PCR,
BACTEC Culture and ZN Smear Microscopy
According to New Cases and Previously
Treated Cases
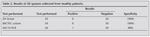
All specimens were colleted from suspected case of extra pulmonary tuberculosis were found to be AFB positive were 60 (13.4%). On the basis of cases, we found that sensitivity of AFB staining on EPTB were 37 (11.2%) in new cases and 23 (18.8%) in previously treated cases. The sensitivity of AFB staining was higher in comparison to previously treated cases. Overall detection rate of M. tuberculosis by AFB Staining was 60 (13.4%). The detection of M. tuberculosis by BACTEC culture was 202 (45%). Results of BACTEC culture according to cases, 151 (46.03%) were in new cases and 51 (41.8%) were in previously treated cases. We found that sensitivity of BACTEC culture was higher in new cases. All culture isolates obtained were confirmed as mycobacteria with biochemical tests mentioned. Using IS 6110 PCR, 283 (61.8%) were positive for IS6110 PCR for M. tuberculosis. 203 (61.8%) were positive in new cases and 80 (65.5%) were positive in previously treated cases. We found that sensitivity of IS6110 PCR was higher in previously treated cases. Overall comparison of tests, IS6110 PCR was found to have much higher sensitivity of 61.8% (Table 1). Among 50 healthy controls, all the samples were negative for smear as well as culture, while only 1 out of these 50 was PCR positive for M. tuberculosis. Specificity was found to be 100% by ZN Staining and BACTEC culture but IS6110 PCR was showed specificity of 98% (Table 2).
Comparison of Sensitivity of IS6110 PCR Test Via
Others Conventional Tests According to New Cases
and Previously Treated Cases
IS6110 PCR test was found to be much more sensitive than ZN staining and BACTEC culture results individually as well as in combination are shown in (Table 3). IS6110 PCR test was found to be much more sensitive than ZN staining and BACTEC culture (p< 0.05). We have compared of sensitivity of IS6110 PCR test via others conventional tests according to new cases and previously treated cases. Of these new cases 328, 37 specimens were positive with AFB and PCR had positive (100%), 127 (56.3%) were PCR positive with smear negative specimens, 147 (97.3%) specimens were BACTEC positive and PCR has positive for M. tuberculosis, 23 (100%) specimens were both positive with AFB smear and BACTEC culture but PCR had positive in 23 (100%), 128 specimens were negative with AFB smear and BACTEC culture was positive but PCR had positive in 117 (91.4%), 14 specimens were positive with AFB smear but BACTEC culture was negative but PCR was positive in 6 (42.8%). In previously treated cases 122, 23 patients were positive with AFB and PCR had positive (100%), 57 (57.5%) were PCR positive with smear negative specimens, 50 (98.1%) specimens were BACTEC positive and PCR has positive, 37 specimen were negative with AFB smear and BACTEC culture was positive but PCR had positives in 35 (94.5%), 5 specimens were AFB smear positive and BACTEC culture were negative but only PCR had positive in 3 (60%). In 225 specimens negative by conventional tests used, PCR test was able to detect 57/163 (34.9%) positives in new cases and 24/68 (38.7%) positives in previously treated cases. these were not likely to represent false positive results as PCR repeat was positive in these specimens and its belonged to highly suspected of EPTB who responded to antitubercular treatment. We found that sensitivity of PCR with ZN staining was not different in new cases and previously treated cases. Our results shown the sensitivities of PCR were higher in previously treated cases as comparison to new cases (Table 3). Studies have reported average detection time for M. tuberculosis was 13.37 days in EPTB cases (25). Our result shown average detection time for M. tuberculosis was 14.76 days by BACTEC and less than one day by PCR test.
Discussion
Tuberculosis (TB) is a major public health dilemma in India. India is the highest TB burden country accounting for one fifth of the global incidence. Global annual incidence estimate is 9.4 million cases out of which it is estimated that 1.98 million cases are from India (26). In India, EPTB comprises 20% of all TB cases. Its prevalence in the country varies between 8.3-13.1% in different districts according to cohort analysis by Central TB Division, Ministry of Health and Family Welfare in 2002 (27,28). The diagnosis of extrapulmonary tuberculosis is till now challenging for diagnostic routine laborites. Numeric reasons are showing that, lack of adequate specimens amounts or volumes; distribute of the specimens for different diagnostic tests (histology/cytology, biochemical analysis, microbiology, and PCR), non-uniform distribution of microorganisms; paucibacillary nature of the specimens; presence of inhibitors that undermine the performance of nucleic acid amplification-based techniques; and the lack of an efficient sample processing technique universally applicable on all types of extrapulmonary samples (29). The poor performance of conventional M. tuberculosis detection techniques, based on microscopic examination of Ziehl-Neelsen stained and culture of M. tuberculosis (LJ Medium and BACTEC Radiometric culture) are still in widespread use for diagnostic purposes, still though they fail to provide the required sensitivity and specificity. The PCR test would be particularly useful in the diagnosis of EPTB where conventional microbiological techniques for M. tuberculosis are showing poor performance of sensitivity. The specificity, sensitivity and speed of PCR test in diagnosis of M. tuberculosis infection shown in this study should encourage the use of this method in routine diagnosis of EPTB.
Previously studies shown the success of microscopy is highly variable from 22% to 96% and most authors rate it at round 60% (30,31,32). Our results shown that sensitivity of smear microscopy was 13.7% and specificity was 100%. The sensitivity of microscopy depends on the clinical presentation and more than 10.000 bacilli per milliliter are necessary for secure microscopic positivity (33). Our studies shown that conventional bacteriological technique were positive in 202 (45%) specimens, where as IS6110 PCR showed that 283 (63%) specimens were positive for M. tuberculosis. The difference was found that to be statistical significant (p< 0.05). Several studies have been reported on PCR to detect M. tuberculosis (34,35,36,37,38,39). The detection of the IS6110 insertion element present in multiple copies to detect M. tuberculosis complex, but not other mycobacterial species (9,40). This fact is increased sensitivity afforded by the detection of the IS6110 insertion sequence considerably improves the yield for the detection of mycobacterial DNA in EPTB cases. Studies reported form documented increased positivity by PCR targeting IS6110 in specimens of EPTB. Sekar et al. showed 63% of positivity, Negi et al. showed that 73%, Tiwari et al. showed 62% of positivity rate among EPTB (12,41,42). But we found that IS6110 PCR positivity was 63% among new and previously treated cases of EPTB. The overall sensitivity of PCR for detecting EPTB greatly exceeded the sensitivity of the acid-fast smear (13.5%) and culture (45%) techniques alone, and the sensitivity of smear and culture used together (72%). PCR sensitivity was considerably better than the conventional methods in all the clinical specimens of EPTB examined. PCR test detected M. tuberculosis is less than one day, compared to average 12.89 to 13.37 days by radiometric BACTEC technique in pulmonary TB and EPTB cases, as supported by earlier studies (25,43). Our studies had shown 14.76 days by radiometric BACTEC technique and PCR detected for M. tuberculosis was less than one day for EPTB cases. Negi et al.? shown that 62.5% were PCR positive with smear negative specimens, 97.5% specimens were BACTEC positive and PCR has positive, 97.8% specimen were negative with AFB smear and BACTEC culture was positive but PCR had positives (41). Therefore our study results on the basis of cases shown that 56.3% were PCR positive with smear negative specimens in new cases and but 57.5% were in previously treated cases. 97.3% specimens were BACTEC positive and PCR has positive in new cases and but 98.1% in previously treated cases. 91.4% specimens were negative with AFB smear and BACTEC culture was positive but PCR had positives in new cases and 94.5% were in previously treated cases. We found that ZN smear examination and PCR results were positive but BACTEC culture was negative; these could be the presence of nonviable mycobacteria in the sample as patients were receiving antitubercular treatment. IS6110 PCR test is higher sensitivity than microscopy and the culture and could help in therapeutic decision for patients with clinical suspicion of EPTB.
Conclusion
IS6110 PCR test for DNA specific M. tuberculosis may be hopes of a rapid and accurate diagnostic test for EPTB and it will help where conventional diagnosis fails and provisional diagnosis of tuberculosis is made on the basis of clinical presentation and histology/cytology examination without evidence of AFB. IS6110 PCR may be great potential to improve the clinician vision for the early diagnosis, treatment and prevention of EPTB.
Acknowledgement
This work was supported by grant from Indian Council of Medical Research, New Delhi (Extramural ICMR Project Sanction No. 5/8/5/4/2007-ECD-I). Authors would like thanks to Technical Members (Mr. Hari Om Verma and Ms. Jyoti Umaro) of Mycobacteriology Laboratory, Department of Microbiology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow, India for technical support during research work.
CONFLICT of INTEREST
None declared.
REFERENCES
- Kant L. Improving detection of infectious cases. Indian J Tuberc 2001; 48: 115-6.
- Pahwa R, Hedau S, Jain S, Jain N, Arora VM, Kumar N, et al. Assessment of possible tuberculous lymphadenopathy by PCR compared to non-molecular methods. J Med Microbiol 2005; 54: 873-8. [?zet] [Tam Metin] [PDF]
- Sharma SK, Mohan A. Extrapulmonary tuberculosis - Review article. Indian J Med Res 2004; 120: 316-53. [?zet] [PDF]
- Roth A, Schaberg T, Mauch H. Molecular diagnosis of tuberculosis: current clinical validity and future perspectives. Eur Resp J 1997; 10: 1877-91. [?zet] [PDF]
- Clarridge JE, Shawar RM, Shinnik, TM, Plikaytis BB. Large scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in routine mycobacteriology laboratory. J Clin Microbiol 1993; 31: 2049-56. [?zet] [PDF]
- Ehlers S, Ingnatius R, Regnath T, Hahn H. Diagnosis of extrapulmonary tuberculosis by Gen-Probe amplified Mycobacterium tuberculosis direct test. J Clin Microbiol 1996; 34: 2275-97. [?zet] [PDF]
- Noordhoek GT, Kolk AHJ, Bjune G, Catty D, Dale JW, Fine PE, et al. Sensitivity and specificity of PCR for detection of Mycobacterium tuberculosis: a blind comparison study among seven laboratories. J Clin Microbiol 1994; 2: 277-84. [?zet] [PDF]
- Rocco MTL, Wagner A, Ocera H, Macias E. Evaluation of a commercial rRNA amplification assay for direct detection of Mycobacterium tuberculosis in processed sputum. Eur J Clin Microbiol Infect Dis 1994; 13: 726-31. [?zet]
- Eisenach KD, Cave MD, Bates JH, Crawford JT. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis 1990; 161: 977-81. [?zet]
- Thierry D, Cave MD, Eisenach KD, Crawford JT, Bates JH, Gicquel B, et al. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res 1990; 18: 188-9. [PDF]
- Hermans PWM, van Soolingen D, Dale JW, Schuitema ARJ, McAdam RA, Catty D, et al. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol 1990; 28: 2051-8. [?zet] [PDF]
- Lahiri KK, Goorha YK. DNA amplification of a repetitive sequence: IS6110 in the early diagnosis of extra pulmonary tuberculosis. Medical Journal Armed Forces India 2001; 57: 12-5.
- Thierry D, Cave MD, Eisenach KD. IS6110 IS-like element of Mycobacterium tuberculosis complex. Nucl Acids Res 1990; 18: 188-92. [?zet] [PDF]
- Carpentier E, Drouillard B, Dailloux M, Moinard D, Vallee E, Dutilh B, et al. Diagnosis of tuberculosis by Amplicor Mycobacterium tuberculosis test: a multicenter study. J Clin Microbiol 1995; 33: 3106-10. [?zet] [PDF]
- Chan CM, Yuen KY,? Chan KS, Yam WC,? Yim KH, Ng WF, et al. Single-tube nested PCR in the diagnosis of tuberculosis. J Clin Pathol 1996; 49: 290-4. [?zet] [PDF]
- Chin DP, Yajko DM, Hadley WK, Sanders CA, Nassos PS, Madej JJ, et al. Clinical utility of a commercial test based on the polymerase chain reaction for detecting Mycobacterium tuberculosis in respiratory specimens. Am J Respir Crit Care Med 1995; 151: 1872-7. [?zet]
- Pfyffer G, Kissling P, Jahn EMI, Welscher H, Salfinger M, Weber R. Diagnostic performance of amplified Mycobacterial tuberculosis direct test with cerebrospinal fluid,other nonrespiratory, and respiratory specimens. J Clin Microbiol 1996; 34: 834-41. [?zet] [PDF]
- Clarridge III JE, Shawar RM, Shinnick TM, Plikaytis BB. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine Mycobacteriology laboratory. J Clin Microbiol 1993; 31: 2049-56. [?zet] [PDF]
- Forbes BA, Hicks KES. Direct detection of Mycobacterium tuberculosis in respiratory specimens in a clinical laboratory by polymerase chain reaction. J Clin Microbiol 1993; 31: 1688-94. [?zet] [PDF]
- Noordhoek GT, Kaan JA, Mulder S, Wilke H, Kolk AHJ. Application of the polymerase chain reaction in a routine microbiology laboratory for detection of Mycobacterium tuberculosis in clinical samples. J Clin Pathol 1995; 48: 810-4. [?zet] [PDF]
- Baron EJ, Finagold SM. Mycobacteria. Baily and Scott?s Diagnostic Microbiology. 9th ed. St Luis: Mosby Company, 1994: 590-33.
- Siddiqui SH. BACTEC 460 TB system. Product and procedure manual. Becton Dickinson Microbiology System. Sparks, MD. 1996.
- Somerville W, Thibert L, Schwartzman K, Behr MA. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J Clin Microbiol 2005; 43: 2996-7. [?zet] [Tam Metin] [PDF]
- Hasaneen NA, Zaki ME, Shalaby HM, Ahmad S. Polymerase chain reaction of pleural biopsy is a rapid and sensitive method for the diagnosis of tuberculous pleural effusion. Chest 2003; 124: 2105-211. [?zet] [Tam Metin] [PDF]
- Khosla R, Dwivedi A, Sarin BC, Sehajpal PK. Peripheral blood based C-PCR assay for diagnosing extra-pulmonary tuberculosis. Indian J Exp Biol 2009; 47: 447-53.
- Global Tuberculosis Control: Epidemiology, Strategy, Financing. Geneva, Switzerland: World Health Organization. WHO/HTM/TB/2009.411 WHO/ HTM/TB/2009.411. [?zet]
- Arora VK, Chopra KK. Extra pulmonary tuberculosis. Indian J Tuberc 2007; 54: 165-7.
- Wares F, Balasubramanian R, Mohan A, Sharma SK. Extra pulmonary tuberculosis, management and control. In: Wares, F, Balasubramanian R, Mohan A, Sharma SK (eds). Agarwal and L. S. Chauhan?s Tuberculosis Control India: Elsevier, 2005: 95-114.
- Chakravorty S, Kamal Sen M, Sivaswami Tyagi J. Diagnosis of extrapulmonary tuberculosis by? smear, culture, and PCR using universal sample processing technology. J Clin Microbiol 2005; 43: 4357-62. [?zet] [Tam Metin] [PDF]
- Querol JM, Farga MA, Granda D, Gimeno C, Garc?a-de-Lomas J. The utility of polymerase chain reaction (PCR) in the diagnosis of pulmonary tuberculosis. Chest 1995; 107: 1631-5. [?zet] [PDF]
- Boyd JC, Marr JJ. Decreasing reliability of acid-fast smear techniques for detection of tuberculosis. Ann Intern Med 1975; 82: 489-92. [?zet]
- Narain R, Rao MS, Chandrasekhar P, Pyarelal. Microscopy positive and microscopy negative cases of pulmonary tuberculosis. Am Rev Respir Dis 1971; 103: 761-7.
- Levy H, Feldman C, Sacho H, van der Meulen H, Kallenbach J, Koornhof H. A reevaluation of sputum microscopy and culture in the diagnosis of pulmonary tuberculosis. Chest 1989; 95: 1193-9. [?zet] [PDF]
- Pao CC, Yen TS, You JB, Maa JS, Fiss EH, Chang CH. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microbiol 1990; 28: 1877-80. [?zet] [PDF]
- De Wit D, Steyn L, Shoemaker S, Sogin M. Direct detection of mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol 1990; 28: 2437-41. [?zet] [PDF]
- Hermans PW, Schuitema AR, Van Soolingen D, Verstynen CP, Bik EM, Thole JE, et al. Specific detection of mycobacterium tuberculosis complex stains by polymerase chain reaction. J Clin Microbiol 1990; 28: 1204-13. [?zet] [PDF]
- Kim HJ, Hyun IK, Lee MK, et al. Diagnosis of tuberculous cervical lymphadenitis using polymerase chain reaction. Tuberc Respir Dis 1995; 42: 35-41.
- Kuwano K, Minamide W, Kusunoki S, Igimi H, Fujiki T, Matsuba K, et al. Evaluation of nested polymerase chain reaction for detecting mycobacterial DNA in pleural fluid. Kansenshogaku Zasshi 1995; 69: 175-80. [?zet]
- Aslansadeh J, Viuda M, Fille M, Smith WB, Namdari H. Comparison of culture and acid-fast bacilli stain to PCR for detection of Mycobacterium tuberculosis in clinical samples. Mol Cell Probes 1998; 12: 207-11. [?zet]
- Thierry D, Cave MD, Eisenach KD, Crawford JT, Bates JH, Gicquel B, et al. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res 1990; 18: 188-9.
- Negi SS, Khan SF, Gupta S, Pasha ST, Khare S, Lal S. Comparison of the conventional diagnostic modalities, bactec culture and polymerase chain reaction test for diagnosis of tuberculosis. Indian J Med Microbiol 2005; 23: 29-33. [?zet]
- Tiwari V, Jain A, Verma RK. Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary and extra-pulmonary tuberculosis. Indian J Med Res 2003; 118: 224-8. [?zet]
- Pfyffer GE, Welscher HM, Kissling P, Cieslak C, Casal MJ, Gutierrez J, et al. Comparison of the Mycobacteria Growth Indicator Tube (MGIT) with radiometric and solid culture for recovery of Acid fast bacilli. J Clin Microbiol 1997; 35: 364-36. [?zet] [PDF]
Yaz??ma Adresi (Address for Correspondence):
Dr. Surya Kant,
Chhatrapati Shahu Ji Maharaj Medical University UP
(Erstwhile King George Medical College),
Lucknow - INDIA
e-mail: dr.kantskt@rediffmail.com