A case of pulmonary embolism confirmed by endobronchial ultrasound
Erdo?an ?ET?NKAYA1, Ayd?n YILMAZ2, Akif ?ZG?L1, Seda ONUR1, Atayla GEN?O?LU1, Sedat ALTIN1
1 SB Yedikule G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi, ?stanbul,
2 SB Atat?rk G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi, Ankara.
Dear Editor,
Endobronchial ultrasonography (EBUS) offers important contributions in sampling of mediastinal and hilar lymph nodes, and in evaluation of esophageal invasions, such as to the vena cava, main pulmonary arteries, that are difficult to diagnose by conventional radiological methods (1). EBUS provides valuable information in interventional bronchoscopy procedures as well as in the diagnosis and staging. In decision-making for curative endobronchial treatment of early lung cancer, the tumor must be limited bounded by the wall. EBUS helps in determination of the best treatment modality because it allows for detailed analysis of the layers of the bronchial wall with its high resolution (2). Recent literature reveals two reports on the efficiency of EBUS in detection of pulmonary thromboembolism (3,4). In our patient a mass in the right hilar region and mediastinal lymphadenopathy was visualized and diagnostic transbronchial needle aspiration was performed in the guidance of EBUS (EBUS-TBNA). Moreover, the thrombus in the right pulmonary artery that was observed on the CT pulmonary angiography was confirmed through EBUS.
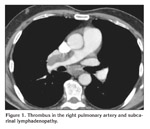
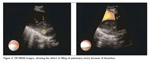
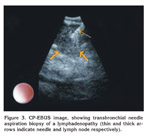
A 63-year-old male patient presented with acute dyspnea and pain in his right chest. Chest radiography revealed fullness in the right hilar region. Arterial blood gase sampling revealed respiratory alkalosis and hypoxemia. Plasma D-dimer level (quantitative) was high (5.12 mg/L). Bedside echocardiography revealed enlarged right cardiac chambers, left ventricle hyperthrophy; diastolic dysfunction in the left ventricle, severe pulmonary hypertension. Systolic pulmonary arterial pressure was 110 mmHg. The patient was suspected of having pulmonary thromboembolism and was evaluated by contrast-enhanced, computed tomography-angiography of the chest (angio-CT). In the evaluation, the following findings were determined: a hypodense lesion of approximately 3 cm in diameter that was limited to a lobule in the right hilus; multiple lymphadenopathy, the largest of which was 23 mm in diameter, in the right lower paratracheal and subcarinal locations, and a thrombus that was 18 mm thick and adherent to the anterior wall in the right main pulmonary artery. The border between the thrombus and the soft tissue of the right hilar could not be discriminated (Figure 1). After ten days of low molecular weight heparine treatment, patient became hemodynamically stable. Arterial blood gas (collected in room temperature) analysis after the treatment was pH: 7.40, PaCO2: 39.2, PaO2: 67 and oxygen saturation: 94%. After that, to obtain diagnostic samples from the lymphoadenopathies and to evaluate the relationship between the soft tissue of the right hilar and the right pulmonary artery, we performed convex probe (CP) endobronchial ultrasound (EBUS). Pulmonary arteries (PA) were echo free. On the image of the embolus in the right PA, an echogenic mass that was surrounded by blood flow and floating within the wall was visualized. The blood flow surrounding the embolus was easily seen by the power doppler mode of the EBUS bronchoscope (model BF 240, EU-ME1 Olympus, Tokyo, Japan) (Figure 2). There was also a heterogenous lymph node with irregular borders. However, the border between this lymph node and the right pulmonary artery was definite. In addition, a homogenous, vascular, lobulated lymphadenopathy with regular contours in the subcranial area and a heterogenous, irregularly contoured lesion extending from the right lower paratracheal area to the upper paratracheal area were detected. Histopathological evaluation of the fine needle aspiration from the subcranial lymphadenopathy and the lesion showed adenocarcinoma (Figure 3).
Conventional pulmonary angiography is considered a gold standart in PTE because it provides definitive diagnosis. The mortality rate with conventional angiography has been reported to be 0.5%, and major morbidity, 1%. Thus, conventional angiography procedures are avoided when possible (5). CT angiography can directly show the thrombus in the pulmonary artery at a segment level. With increased number of detectors (≥ 4), the sensitivity of spiral CT in detecting subsegments of and beyond peripheral thrombi increases (6). CT angio is the most common method for diagnosis of pulmonary embolism (7). CT-angio is counterindicated in those with renal failure and allergy to contrast matter. Although it is not counterindicated, exposure to radiation should be avoided during pregnancy. In the study PIOPED II, in 24% of the patients with suspected acute PE, one or more counterindications were found (6).
With CP-EBUS, the pulmonary arteries around the central airways can be evaluated (3,4). Casoni et al. used EBUS in distinguishing intraarterial appearance of low density in the right pulmonary artery from a suspected right pulmonary artery sarcoma detected on pulmonary angiography. They imaged an embolism in the right main pulmonary artery that was not infiltrating the arterial wall and established a definitive diagnosis of pulmonary thromboembolism by using EBUS (3). In our patient, the power mode of EBUS easily distinguished the embolism surrounded by blood circulating in the artery, and the diagnosis of pulmonary embolism was confirmed.
Aumiller et al. in their multicenter prospective study, evaluated the cases in whom they had detected central PE by CT-angio by CP-EBUS within 24 hours. They compared EBUS images and CT findings. In 32 patients, 101 PE were detected by CT-angio and 97 of these PEs were confirmed by EBUS. Because they had determined at least one embolism in each patient, EBUS was considered effective in confirming the diagnosis of central PE. The authors did not encounter any bronchoscopy associated complications and reported EBUS as a reliable and safe method of diagnosis for central PE (4).
Ventilation/perfusion (V/Q) lung scintigraphy requires the use of radioactive materials. With increasing age and in the presence of chronic disease such as COPD, the rate of non-diagnostic scintigraphy increases (8,9). The value of contrast-enhanced CT pulmonary angiography in the diagnosis of pulmonary embolism is indisputable (10). However, its use in those with renal failures and allergy to contrast matter is counterindicated. It also has a limited use in pregnant individuals because of radiation exposure (6). EBUS can be performed with the patient under local anesthesia and conscious sedation. It does not require contrast-matter use or radiation exposure. Endobronchial ultrasonography might assume its place in diagnostic algorithm for pulmonary embolism, particularly for patients in whom contrast-matter use is counterindicated.
The purpose of this letter is not to give a place to EBUS in the diagnosis of pulmonary embolism, rather that, we suggest that during an EBUS session, great arteries in the mediastinum must carefully be evaluated in order to detect incidental pathologies. Embolus in the proximal portions of great arteries could be clearly demonstrated by EBUS. But this approach must be limited to very selected, hemodinamically stable patients.
CONFLICT of INTEREST
None declared.
REFERENCES
- Herth F, Ernst A, Schulz M, Becker H. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003; 123: 458-62. [?zet] [Tam Metin] [PDF]
- Herth F, Becker HD. EBUS for early cancer detection. J Bronchol 2003; 10: 249-53.
- Casoni GL, Gurioli C, Romagnoli M, Poletti V. Diagnosis of pulmonary thromboembolism with endobronchial ultrasound Eur Respir J 2008; 32: 1416-7. [Tam Metin] [PDF]
- Aumiller J, Herth FJ, Krasnik M, Eberhardt R. Endobronchial ultrasound for detecting central pulmonary emboli: a pilot study. Respiration 2009; 77: 298-302. [?zet]
- Stein PD, Athanasoulis C, Alavi A, Greenspan RH, Hales CA, Saltzman HA, et al. Complications and validity of pulmonary angiography in acute pulmonary embolism. Circulation 1992; 85: 462-8. [?zet] [PDF]
- Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multi-detector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: 2317-27. [?zet] [Tam Metin] [PDF]
- Resten A, Mausoleo F, Valero M, Musset D. Comparison of doses for pulmonary embolism detection with helical CT and pulmonary angiography. Eur Radiol 2003; 13: 1515-21. [?zet]
- Scarsbrook AF, Bradley KM, Gleeson FV. Perfusion scintigraphy: diagnostic utility in pregnant women with suspected pulmonary embolic disease. Eur Radiol 2007; 17: 2554-60. [?zet]
- Chan WS, Ray JG, Murray S, Coady GE, Coates G, Ginsberg JS. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med 2002; 162: 1170-5. [?zet] [Tam Metin] [PDF]
- Fedullo PF, Tapson VF. The evaluation of suspected pulmonary embolism. N Engl J Med 2007; 349: 1247-56.
Yaz??ma Adresi (Address for Correspondence):
Dr.
Ayd?n YILMAZ
SB Atat?rk G???s
Hastal?klar? ve
G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
7. Servis
Sanatoryum, Ke?i?ren
06280 ANKARA - TURKEY
e-mail: aydnylmaz@yahoo.com