Pulmoner tromboemboliyi taklit eden pulmoner arter sarkomu
G?khan
?el?K1, Ayd?n ??leda?1, Cabir Y?ksel2, B?lent Mustafa Yen?g?n2, Hakan Kutlay2,
Levent YazIcIo?lu3, Sibel Per??nel4, Ak?n Kaya1
1 Ankara ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Ankara,
2 Ankara ?niversitesi T?p Fak?ltesi, G???sCerrahisi Anabilim Dal?, Ankara,
3 Ankara ?niversitesi T?p Fak?ltesi, Kardiyovask?ler Cerrahi Anabilim Dal?, Ankara,
4 Ankara ?niversitesi T?p Fak?ltesi, Patoloji Anabilim Dal?, Ankara.
?ZET
Pulmoner tromboemboliyi taklit eden pulmoner arter sarkomu
Otuz ya??nda erkek hasta, toraks bilgisayarl? tomografi (BT) anjiyografide sol ana pulmoner arterde l?meni tam t?kayan tromb?s saptanmas? ?zerine pulmoner emboli tan?s? ile hastanemize refere edildi. Klini?imizde, lezyon t?m?ral kitle olarak de?erlendirildi. Pozitron emisyon tomografi (PET)-BT'de, pulmoner arterdeki kitlede patolojik tutulum saptand?. T?m?r?n lokalizasyonu nedeniyle sol pn?monektomi uyguland?. Patolojik incelemede pulmoner arter sarkomu saptand?. Olgu, pulmoner arter sarkomunun nadir g?r?lmesi ve pulmoner emboliyi taklit edebilmesi nedeniyle sunuldu. Pulmoner arter sarkomunun ger?ek prevalans?, bir?o?unun yanl??l?kla pulmoner tromboemboli tan?s? almas? nedeniyle tahmin edilenin ?zerindedir. PET-BT ay?r?c? tan?da yard?mc? olabilir.
Anahtar Kelimeler: Pulmoner tromboemboli, pulmoner arter sarkomu, cerrahi.
SUMMARY
Pulmonary artery sarcoma mimicking pulmonary thromboembolism
G?khan
?el?K1, Ayd?n ??leda?1, Cabir Y?ksel2, B?lent Mustafa Yen?g?n2, Hakan Kutlay2,
Levent YazIcIo?lu3, Sibel Per??nel4, Ak?n Kaya1
1 Department of Chest Diseases, Faculty of Medicine, Ankara University, Ankara, Turkey,
2 Department of Chest Surgery, Faculty of Medicine, Ankara University, Ankara, Turkey,
3 Department of Cardiovascular Surgery, Faculty of Medicine, Ankara University, Ankara, Turkey,
4 Department of Pathology, Faculty of Medicine, Ankara University, Ankara, Turkey.
A 30 years old male patient was referred to our hospital with a diagnosis of pulmonary thromboembolism due to thorax-computerized tomography (CT) angiography, revealing a thrombus totally occluding left main pulmonary artery. The lesion was evaluated as tumoural mass. Positron emission tomography (PET)-CT revealed pathologic uptake at pulmonay artery mass. Due to localization of tumour, left pneumonectomy was performed. The pathological diagnosis revealed to be pulmonary artery sarcoma. The patient was presented because pulmonary artery sarcomas are very rare tumors and can mimick pulmonary thromboembolism. The true prevalence is underestimated as many pulmonary artery sarcomas are misdiagnosed as pulmonary thromboembolism. PET-CT may help to make a differential diagnosis.
Key Words: Pulmonary thromboembolism, pulmonary artery sarcoma, surgery.
Pulmonary artery sarcoma (PAS) or intimal sarcoma (IS) of the pulmonary artery is a rare malignancy arising from the mesenchymal cells of the intima of the pulmonary artery. The diagnosis is difficult and often delayed owing to the nonspecific nature of the symptoms. Since PAS is very rare and insidious, it is frequently misdiagnosed as pulmonary thromboembolism. The differentiation of these two entities is fundamental to avoid inappropriate therapy, such as prolonged anticoagulation or thrombolysis. Although the survival of patients with PAS is very short, it may be prolonged if the tumor is diagnosed early and appropriate treatment is applied.
CASE REPORT
A 30-years-old male patient had admitted to another hospital with cough and pleuritic chest pain. Thorax computerized tomography (CT) had revealed consolidation at left lower lobe and he had been treated with antibiotics. After partial improvement, because of worsening of complaints and development of dyspnea, he had admitted to emergency department. Thorax CT angiography had been performed and a thrombus totally occluding the left main pulmonary artery had been detected. Unfractioned heparin infusion had been initiated. Consequently, the patient was referred to our clinic with a diagnosis of pulmonary thromboembolism.
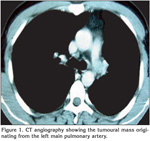
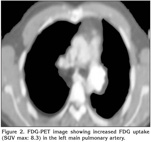
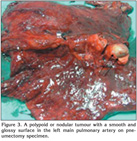
On physical examination, his temperature was 36.5?C, the respiratory rate 16/min, heart rate 87/min, rhythmic, and blood pressure being 110/60 mmHg. Chest examination revealed rales at the left hemithorax. The physical examination was otherwise normal. Laboratory studies revealed a normal complete blood count and serum biochemistry. The coagulation tests and D-dimer level were in normal range. The CT-angiography images were checked and extraluminal expansion of lesion was observed (Figure 1). The lesion was evaluated as a tumoural mass originating from artery wall approximately 1 cm to exit of the left main pulmonary artery. Heparin was stopped. Positron emission tomography (PET)-CT revealed pathologic 18 FDG uptake at pulmonay artery mass with SUV max of 8.3 (Figure 2). A transthoracic echocardiography (ECHO) revealed a pulmonary artery systolic pressure of 45 mmHg and minimal tricuspid insufficiency, otherwise the ECHO examination was normal. Due to the localization of the tumour, left thoracotomy and pneumonectomy was performed. Macroscopically, a polypoid or nodular sessile lesion having a smooth and glossy surface protruded into the lumen of the left main pulmonary artery on left pneumonectomy (Figure 3). The lesion had a size of 20 x 20 x 16 mm and was solid in consistency, grayish white in color, with areas of haemorrhage.
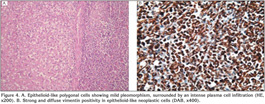
Microscopically, a cellular lesion totally obliterating the lumen of the artery was seen. It attached and partly invaded the intima without invading the wall of the artery or penetrating into the surrounding connective tissue. The lesion which displayed diffuse pattern in a myxoid and edematous background was mainly composed of epithelioid-like polygonal cells having vesicular, round-oval nuclei, eosinophilic or clear cytoplasm without clear-cut boundries showing mild pleomorphism and sparse mitotic activity. Occasional spindle cells were also observed. An intense plasma cell infiltration and Russel bodies surrounding the polygonal and spindle cells were detected (Figure 4A). No necrosis was observed in the lesion.
Immunohistochemically, the cells which made up the lesion were strongly and diffusely positive only for vimentin (Figure 4B). They did not stain for pan-cytokeratin, high and low molecular weight cytokeratins, cytokeratin 7 and 20, epithelial membrane antigen, CD10, renal cell carcinoma marker, desmin, h-caldesmon, smooth muscle actin, thyroid transcription factor-1, CD34, fli-1, chromogranin-A, synaptophysin, S-100, CD68, or myogenin.
On the basis of clinical, radiological, morphological and immunohistochemical findings, the lesion was diagnosed as the the low grade PAS or IS. No adjuvant therapy was administered.
DISCUSSION
Intimal sarcoma of pulmonary artery, first described by Mandelstamm in 1923, is a rare and potentially lethal tumor, usually diagnosed during surgery or autopsy (1). It is thought to arise from multipotential mesenchymal cells of the intima and generally arises from the right, left and main pulmonary artery. The reported age ranges from 13 to 86 years with the majority of cases occuring in the middle age. Although some studies revealed female predominance, more recent data suggest no gender preference (2).
Clinical symptoms are generally non-specific and often occur at the end-stage of the disease. Frequent symptoms are dyspnea, chest pain, hemoptysis, weight loss, malaise, cough, syncope, and fever.
Although PAS is a rare tumour, the true prevalence is underestimated as many cases of PAS are misdiagnosed as pulmonary thromboembolism leading to inappropriate therapy. Both diseases appear as intraluminal filling defects in the pulmonary artery system on contrast-enhanced CT scans and differentiating radiologically between PAS and pulmonary artery thromboembolism is relatively difficult. However, over the last decades, improvement of imaging techniques has allowed to distinguish the two entities before surgery. Yi et al. reported that, CT findings favoring the diagnosis of PAS included a low attenuation filling defect occupying the entire luminal diameter of the proximal or main pulmonary artery, expansion of the involved arteries and extraluminal tumour extension as shown in our case (3). In addition, PAS has more likely a heterogenous appearance with areas of necrosis, haemorrhage, and ossification. It occurs frequently unilaterally, in contrast with the often bilateral involvement of pulmonary arteries in the thromboembolic disease.
Recently, it has been reported that, PET-CT may help to make a differential diagnosis between PAS and pulmonary artery thromboembolism (4). According to the several case reports on the cardiovascular application of FDG-PET, blood thrombi showed negative FDG uptake, whereas a malignant tumour, such as a cardiac sarcoma showed positive FDG uptake. FDG-PET may show intense tracer activity within a PAS. While, some tracer activity within a thromboembolic disease may occur, intense tracer localization within a central pulmonary arterial filling defect strongly suggests primary pulmonary arterial malignancy (5). We think that, the positive FDG uptake on PET-CT in the current case, supports this suggestion.
The diagnosis of PAS requires a high clinical suspicion. Although biopsy with intravascular forceps during angiography, transvenous catheter suction and CT-guided needle aspiration are possible, the exact diagnosis is often made during surgery as in our case.
IS is listed under the heading of "Tumours of Uncertain Differentiation" in the World Health Organization Classification of Tumours of Soft Tissue and Bone (6). IS of the pulmonary artery has been used interchangeably with PAS by the World Health Organization Classification of Tumours of the Lung, Pleura, Thymus and Heart, under the heading of the mesenchymal tumours of the lung since ISs comprise the vast majority PASs (7).
A sarcoma of the large pulmonary arteries are of two types: intimal and mural sarcomas. ISs have a predominant intraluminal polypoid growth pattern with obstruction of the lumen of the vessel of origin and usually show fibroblastic or myofibroblastic differentiation. Mural sarcomas, exceedingly rare are considered distinct from ISs, and are classified separately according to the histologic subtype as in soft tissue sarcomas (leiomyosarcoma) (7).
Grossly, ISs resemble mucoid or gelatinous clots filling vascular lumens and attached to the vessel wall resembling thrombi. They may extend distally along the branches of the involved vessels. The cut surface may show firm fibrotic areas and bony/gritty areas corresponding to osteosarcomatous or chondrosarcomatous differentiation. Haemorrhage and necrosis are common in high-grade tumours (8).
ISs are usually poorly differentiated mesenchymal malignant tumours of fibroblastic or myofibroblastic differentiation, consisting of mildly to severely atypical spindle cells with varying degrees of atypia, mitotic activity, necrosis and nuclear pleomorphism (6,7,8). Some tumours show large myxoid areas or epithelioid appearance of tumour cells. Tumours may be reminiscent of leiomyosarcoma due to prominent spindling and bundling of the cells. Foci of rhabdomyosarcomatous, osteosarcomatous or angiosarcomatous differentiation may be detected in rare cases. Aortic ISs uncommonly contain areas of specific differentiation other than myofibroblastic one unlike PASs (6,7).
Most ISs show immunohistochemical and ultrastructural evidence of myofibroblastic differentiation. The tumour cells, in general, exhibit strong and diffuse immunoreactivity for vimentin. Osteopontin expression can also be expressed and smooth muscle actin immunoreactivity is variable. The endothelial markers; CD31, CD34, and FVIII which show positivity for angiosarcoma are negative in a typical case of IS (6,7,8).
On the basis of clinical, radiological, morphological and imunophenoytpical findings, the diagnosis can be established in most cases, though some thrombi may have highly cellular foci which cause the main differential diagnostic difficulty. Metastases and primary sarcomas, particularly angiosarcoma should always be excluded from ISs.
The cornerstone of the treatment consists of surgical resection through endarterectomy, tumourectomy or pneumectomy. Surgical resection remains the primary treatment to provide the significant palliation and offers the only chance of cure (1).
The role of adjacent chemotherapy or radiotherapy is uncertain. Previous literature has reported the disease to be uniformly fatal. A mean survival of 1.5 months after diagnosis without surgery versus 10 to 14 months with surgery has been reported in the past (9,10). More recent data suggest that diseases-free survival for greater than three years is possible with histopathologically low-grade lesions. Our patient was alive after one year of surgery without any complaints.
In conclusion, we have reported a very rare case of PAS originating from left pulmonary artery. Although very rare, in case of unilateral main pulmonary artery embolism or with a history of recurrent thromboembolism at same localization, PAS or IS should be considered in the differential diagnosis. PET-CT may help to make a differential diagnosis.
CONFLICT of INTEREST
None declared.
REFERENCES
- Hu XP, Xu JP, Liu NN. Primary pulmonary artery sarcoma: surgical management and differential diagnosis with pulmonary embolism and pulmonary valve stenosis. J Card Surg 2009; 24: 613-6. [?zet]
- Tavora F, Miettinen M, Fanburg-Smith J, Fransk TJ, Burke A. Pulmonary artery sarcoma: a histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. Am J Surg Pathol 2008; 32: 1751-61. [?zet]
- Yi CA, Lee KS, Choe YH, Han D, Kwon OJ, Kim S. Computed tomography in pulmonary artery sarcoma: distinguishing from pulmonary embolic diseases. J Comput Asist Tomogr 2004; 28: 34-9. [?zet]
- Chong S, Kim TS, Kim BT, Cho EY, Kim J. Pulmonary artery sarcoma mimicking pulmonary thromboembolism: integrated FDG PET/CT. AJR 2007; 188: 1691-3. [?zet] [PDF]
- Gotway MB, Tillinghast AJ. Pulmonary artery filling defects on thoracic cross sectional imaging. Clin Pulm Med 2009; 16: 109-13.
- Fletcher CDM , Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. Lyon: International Agency for Research on Cancer Press, 2002: 223-24.
- Travis WD, Brambilla E, M?ller-Hermelink HK, Harris CC. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer Press, 2004: 12-77.
- Nonomura A, Kurumaya H, Kono N, Nakanuma Y, Ohta G, Terahata S, et al. Primary pulmonary artery sarcoma. Report of two autopsy cases studied by immunohistochemistry and electron microscopy, and review of 110 cases reported in the literature. Acta Pathol Jpn 1988; 38: 883-96. [?zet]
- Kruger I, Borowski A, Horst M, de Vivie ER, Theissen P, Gross-Fengels Wl. Symptoms, diagnosis and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg 1990; 38: 91-5. [?zet]
- Parish JM, Rosenow EC, Swensen SJ, Crotty TB. Pulmonary artery sarcoma. Clinical features. Chest 1996; 110: 1480-8. [?zet]
Yaz??ma Adresi (Address for Correspondence):
Dr. Ayd?n ??LEDA?,
Ankara ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
06100 Cebeci, ANKARA - TURKEY
e-mail: aciledag@yahoo.com