Stabil KOAH'l? hastalarda sistemik inflamasyon ve metabolik sendrom
Evrim Eylem AKPINAR1, Serdar AKPINAR2, Sibel ERTEK3, Esen SAYIN1, Meral G?LHAN1
1 Ufuk ?niversitesi T?p Fak?ltesi, G???s Hastal?klar? Anabilim Dal?, Ankara,
2 Atat?rk G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
5. G???s Hastal?klar? Klini?i, Ankara,
3 Ufuk ?niversitesi T?p Fak?ltesi, ?? Hastal?klar? Anabilim Dal?, Ankara.
?ZET
Stabil KOAH'l? hastalarda sistemik inflamasyon ve metabolik sendrom
Giri?: Kronik obstr?ktif akci?er hastal??? (KOAH) sistemik inflamasyonla ili?kili gibi g?r?nen ekstrapulmoner etkilere sahiptir. Genel pop?lasyonda sistemik inflamasyonun ?nemli belirleyicilerinden biri olan metabolik sendromla KOAH aras?ndaki ili?ki hen?z netle?memi?tir. Bu ?al??man?n amac?; farkl? evrelerdeki stabil KOAH'l? hastalarda ve ya?, cinsiyet a??s?ndan e?le?tirilmi? kontrol grubunda metabolik sendrom frekans?n? ve sistemik inflamasyon belirteci olan C-reaktif protein (CRP) d?zeylerini de?erlendirmektir.
Hastalar ve Metod: ?al??maya 91 stabil KOAH'l? hasta ve 42 kontrol birey al?nd?. KOAH a??rl??? GOLD (Global Initiative for Chronic Obstructive Lung Disease) kriterlerine g?re belirlendi. Metabolik sendrom tan?s?nda ATP III (The National Cholesterol Education Program's Adult Treatment Panel III) kriterleri kullan?ld?. Hasta ve kontrol grubunda al?nan ven?z kan ?rne?inde CRP d?zeyleri ?l??ld?.
Bulgular: Metabolik sendrom frekans? hasta grubunda, ?zellikle GOLD I, II'de, kontrol grubundan daha y?ksek bulundu (p= 0.004). Metabolik sendromun abdominal obezite, hipertansiyon ve hiperglisemi komponentlerinin frekans? hasta grubunda daha y?ksek bulundu (p< 0.0001). Artm?? CRP d?zeyleri kontrol grubunda ve hasta grubun t?m evrelerinde, metabolik sendrom olanlarda, olmayanlara g?re daha y?ksek orandayd? (p= 0.047, p= 0.217, p< 0.001, p= 0.05, p= 0.467).
Sonu?: Bu ?al??ma metabolik sendrom frekans?n?n stabil KOAH'l? hastalarda, kontrol grubundan ve T?rk pop?lasyonundan daha y?ksek oldu?unu g?stermi?tir. Abdominal obezite, hipertansiyon ve hiperglisemi hasta grubunda anlaml? derecede daha s?kt?. Sistemik inflamasyon metabolik sendromu olan KOAH'l? hastalarda olmayanlara g?re daha yo?undu.
Anahtar Kelimeler: KOAH, metabolik sendrom.
SUMMARY
Systemic inflammation and metabolic syndrome in stable COPD patients
Evrim Eylem AKPINAR1, Serdar AKPINAR2, Sibel ERTEK3, Esen SAYIN1, Meral G?LHAN1
1 Department of Chest Diseases, Faculty of Medicine, Ufuk University, Ankara, Turkey,
2 Clinic of Chest Diseases, Ataturk Chest Diseases and Chest Surgery Training and Research Hospital,
Ankara, Turkey,
3 Department of Internal Medicine, Faculty of Medicine, Ufuk University, Ankara, Turkey.
Introduction: Chronic obstructive pulmonary disease (COPD) has extrapulmonary effects that seems to be related with systemic inflammation. The relationship between metabolic syndrome which is an important determinant of systemic inflammation in general population and COPD is still not clear. The aim of the current study was to investigate the frequency of metabolic syndrome and C-reactive protein (CRP) levels, as a marker of systemic inflammation in stable COPD patients with different severity levels and in age and sex matched control group.
Patients and Methods: Ninety-one stable COPD patients and 42 control subjects were included in the study. The severity level in patients with COPD were determined according to? GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria. ATP III (The National Cholesterol Education Program's Adult Treatment Panel III) was used in diagnosis of metabolic syndrome. Hs-CRP levels were measured in venous samples of patients and control subjects.
Results: The frequency of metabolic syndrome was found higher in patient group than control subjects, especially in GOLD stages I, II (p= 0.004). Abdominal obesity, hypertension, hyperglycemia components of metabolic syndrome were significantly more prevalent in patient group (p< 0.0001). Increased CRP levels were higher in control and patient groups in all GOLD stages, with metabolic syndrome than without metabolic syndrome (p= 0.047, p= 0.217, p< 0.001, p= 0.05, p= 0.467).
Conclusion: The study showed that frequency of metabolic syndrome was higher in stable COPD patients than control subjects and general Turkish population. Abdominal obesity, hypertension and hyperglycemia were significantly more prevalent in patient group. Systemic inflammation was more intense in COPD patients with metabolic syndrome than without metabolic syndrome.
Key Words: COPD, metabolic syndrome.
Geli? Tarihi/Received: 28/01/2012 - Kabul Edili? Tarihi/Accepted: 31/07/2012
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth-leading cause of chronic morbidity and mortality worldwide. It is characterized by progressive, partially reversible airflow limitation that is associated with an abnormal inflammatory response of lungs to noxious particles or gases, particularly cigarette smoke. Diagnosis of COPD can be established by a fixed ratio of post bronchodilator FEV1 and FVC below 0.7 measured by spirometry. Spirometric severity is graded according to percentage of FEV1 predicted (GOLD stage I-IV) (1). Cigarette smoking is the major risk factor for COPD. It causes not only local inflammation on lungs, but also systemic inflammation that is thought to contribute to the development of chronic diseases as well as COPD, like cardiovascular diseases, hypertension and diabetes (2). There is accumulating evidence that COPD has many extrapulmonary effects thought to be related to systemic inflammation. Clinical severity of the disease is determined not only by spirometry but also by concomitant comorbidities (3,4). The previous studies showed that markers of systemic inflammation like high-sensitivity C-reactive protein (CRP), interleukin (IL)-6 were higher in blood of COPD patients than the ones without COPD (3,5). Whereas, the serum levels of these inflammatory markers did not correlate with their level in sputum. These results have caused us not to hold "the overspill hypothesis" that proposed systemic inflammation caused by pulmonary inflammation through migration of the local mediators and have led to the start of the discussion related to systemic nature of inflammation associated with COPD (6).
Metabolic syndrome (MetS) is characterized by a group of risk factors (abdominal obesity, atherogenic dyslipidemia, raised blood pressure, insulin resistance) that increases the development of several diseases such as coronary artery disease, diabetes mellitus (7,8). It was first described in 1988 by Reaven, also known as "syndrome X". It was defined with the clustering risk factors for cardiovascular disease by means of underlying common path physiological findings. MetS is not a real syndrome. The following ideas were attributed when it was first defined; to start with, one or more risk factors can play a role in the development of diseases simultaneously, like diabetes, obesity, cardiovascular diseases and hypertension. Secondly, diagnosis of chronic disorders needs extensive clinical evaluation. Thirdly, chronic comorbid disorders should be treated concurrently and all risk factors should be eliminated by modifying life style (e.g. weight loss, regular physical activity, smoking cessation) (9). The results of the study including a large number of Chinese population reported by Lam et al. suggested that both the presence of airflow obstruction was related to MetS and the risk increased with the severity of obstruction (10).
COPD is one of the diseases in which smoking is the common and important risk factor when it is associated with MetS. The association between MetS and systemic inflammation has been well documented (11). It has been shown that 50% of patients with COPD had one or more components of the MetS (12). High prevalence (61%) of MetS in men with COPD participating to a pulmonary rehabilitation program, in contrast to lower prevalence (44%) in age-matched men without COPD was reported (13,14). Marquis and colleagues' proposed that an increased prevalence of MetS in patients with COPD may have explained this association (12). Systemic inflammation plays a key role in both COPD and MetS but real inflammatory profile of these patients is still unknown. The aim of this study was to investigate the prevalence of MetS in COPD patients who were in different GOLD stages and control subjects. High sensitive CRP levels were also evaluated in patient and control groups to define the level of systemic inflammation and its correlation with the presence of MetS.
PATIENTS and MethodS
Study Design and Subject Characteristics
The study was designed as a prospective case-control study. Patients with stable COPD (stage I-IV) who were admitted to outpatient clinic of an University Hospital in capital city of Turkey (Ufuk University, Ankara) between August 2010-September 2011 were included in the study. The study had the approval of Ethics Committee of Ufuk University. Informed consents of all patients and control subjects were taken before they were included in the study.
The criteria for exclusion were having an acute exacerbation (increase in cough, sputum production, worsening dyspnea, or sputum purulence within three weeks) (1), having any infectious or inflammatory diseases such as collagen vascular diseases, inflammatory bowel disease that could cause an increase in CRP levels.
The subjects who were selected for control group were smokers or non-smokers, age and sex matched with patient group, with normal spirometry and without any infectious or inflammatory diseases that could increase CRP levels.
Diagnosis of COPD
The diagnosis of COPD was made according to GOLD (Global Initiative for Chronic Obstructive Lung Disease) criteria (1). Since it was planned to be a prospective study, collecting patients was started before the report of GOLD 2011, updated version of GOLD consensus in 2008 was used.
Pulmonary Function Testing
Spirometry was made by using VIASYS Healthcare V max? 20 Pulmonary Spirometry Instrument (Germany, 2009). The staging of COPD was made by using GOLD criteria: GOLD I (mild): FEV1/FVC < 70% and FEV1 ≥ 80%; GOLD II (moderate): FEV1/FVC < 70% and FEV1 < 80% and ≥ 50%; GOLD III (severe): FEV1/FVC < 70% and FEV1 < 50% and ≥ 30%; GOLD IV (very severe): FEV1/FVC < 70% and FEV1 < 30% (1).
Blood Sampling and Analyses
A venous blood sample was collected from each subject after a 12-hour fasting. Blood samples were taken in stable phase of COPD patients. Plasma glucose, triglyceride (TG) and high density lipoprotein (HDL) were measured by using both a Roche COBAS INTEGRA? 400 plus analyzer (Germany, 2009) and an enzymatic calorimetric assay. High sensitivity-CRP levels were measured by using a Roche COBAS INTEGRA? 400 plus analyzer (Germany, 2009) by automatic calorimetric assay, CRP levels which were greater than 5 ?g/L were accepted as "high" otherwise "low".
Diagnosis of MetS
Body weight and height were measured and the body mass index (BMI) was calculated by dividing weight by height squared (kg/m2). Blood pressure was measured according to the American Heart Association's recommendations. Blood pressure measurements were obtained from both arms in the supine position after 15 min resting period and the highest measurement was used for analysis (15). Waist circumference was measured according to the procedures of Airlie Conference (16). ATP III (The National Cholesterol Education Program's Adult Treatment Panel III) was used in diagnosis of MetS (Table 1) (17). If the participants were using antihypertensive or antidiabetic drugs, they were considered to have had high blood pressure or high fasting glucose.
Statistical Analysis
Statistical analyses were carried out with SPSS for Windows version 15.0 statistical software (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ? standard deviation and categorical variables as percentages. Chi-square test was used to determine the associations between categorical variables. Continuous variables were examined for normality by Shapiro Wilks test and homogeneity of variances by Levene test. For normally distributed variables, differences between the groups were determined by independent samples t test. Mann-Whitney U test was used for abnormally distributed variables. Significance value was considered as 0.05.
Results
Ninety-one stable COPD patients and 42 control subjects were included in the study. Demographic properties and smoking history of patients and control subjects were shown on Table 2.
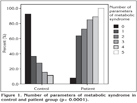
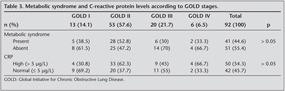
The distribution of COPD patients according to GOLD stages (I-IV) were respectively 14.1%, 57.6%, 21.7% and 6.5%. The prevalence of MetS in patient group was found much higher than control group (44.6% vs. 17.1%) (p= 0.004). The distribution of the prevalence of MetS between GOLD stages I-IV were as follows; 38.5%, 52.8%, 30% and 33.3% respectively. The number of the diagnostic criteria of MetS was found significantly higher in patient group (p< 0.0001) (Figure 1). High sensitive-CRP level increased in 53.8% of COPD patients. Whereas high CRP levels were found only in 26.2% of control group (p= 0.005). High CRP levels seemed to be more prominent in GOLD stage II patients. But the difference was not statistically significant (p= 0.146). The distribution of MetS and increased CRP levels between GOLD stages were shown on Table 3. C-reactive protein levels were higher in both patient and control groups who had MetS than the ones without MetS (85.4% vs. 29.4%, 57.1% vs. 17.6%). The difference between CRP levels in patients with MetS and without MetS was highly significant, whereas the difference in control group was not statistically noticeable (p< 0.0001, p= 0.083, respectively).
The parameters of MetS were evaluated one by one in both patient and control groups. Abdominal obesity, hypertension, hyperglycemia components of MetS were significantly higher in patient group (p< 0.0001).The ratios were 52.2% vs. 14.6%, 77.2% vs. 36.6%, 46.7% vs. 9.8% respectively (p< 0.05 for all). In contrast, triglyceride and HDL components were higher in control group (25% vs. 26.8%, 34.8% vs. 43.9%) but the difference was not significant (p= 0.491, p= 0.209).
CRP levels were higher in patients who had MetS than the ones who did not have MetS in all GOLD stages. Increased CRP levels were also higher in control subjects with MetS than the ones without MetS. The difference was significant only in control group and patients with GOLD stage II and nearly significant in stage III. p values for control group and GOLD stages I-IV were p= 0.047, p= 0.217, p< 0.001, p= 0.05, p= 0.467 respectively (Figure 2).
Discussion
The main findings of the present study were as follows; prevalence of MetS was found higher in stable COPD patients, especially in patients with GOLD stage II, than general Turkish population and age-sex matched control group. Serum CRP levels, as a marker of systemic inflammation, were higher in COPD group than control group. Additionally, patients with MetS had higher CRP levels than COPD patients without MetS. When the parameters of MetS were evaluated one by one; abdominal obesity, hypertension and hyperglycemia were especially more prevalent in patient group.
COPD is characterized by chronic airway inflammation, but there is increasing evidence that the disease is not restricted to the lungs. Smoking is a major risk factor not only for development of COPD but also many other chronic diseases. It triggers a local inflammatory response in lungs and it also causes systemic inflammation that result in comorbidities like cardiovascular or metabolic disorders (18). It is not clear yet whether this inflammation spreads from lung to systemic circulation or multiple organ systems including lung are affected due to a systemic inflammatory response (2,19,20). It was shown that systemic inflammatory markers such as IL-6, IL-8, TNF-α increased in COPD patients particularly who were in acute exacerbation (21,22). The plasma concentrations of CRP, another inflammatory marker, were found higher in stable COPD patients and were thought to be related to the mortality in mild-to-moderate stages of the disease (23,24).
Mets is characterized by a group of risk factors (abdominal obesity, atherogenic dyslipidemia, raised blood pressure, insulin resistance) which are responsible for development of co morbid diseases like cardiovascular diseases and diabetes mellitus. The relationship between MetS and COPD was investigated before but not yet clearly understood (9,12). Smoking that is a major risk factor for COPD causes systemic inflammation besides lung inflammation. However, high prevalence of MetS in COPD patients can not be attributed to smoking alone. Systemic inflammation may play a determinant role in the relationship between MetS and COPD (2).
Watz et al. investigated the prevalence of MetS in COPD patients. They reported average frequency of MetS in this group of patients as 47.5%. Frequencies according to GOLD stages (I-IV) were as follows 50%, 53%, 37%, 44% (9). Similarly, the prevalence of MetS in COPD patients was found 44.6% in our study. The distribution of the prevalence of MetS between GOLD stages I-IV were as follows; 38.5%, 52.8%, 30% and 33.3% respectively. The prevalence in patients with GOLD stage II was the highest in both studies whereas in our study, the frequencies in patients who were in other stages of COPD were lower than the results of the study that was performed by Watz et al. (9). The lower frequencies of MetS in later stages of COPD may be resulted from apparent weight loss in these patients. The higher mortality rates in COPD patients who were associated with MetS due to concomitant diseases like cardiovascular diseases or diabetes mellitus may also be contributed to lower frequencies in later stages of the disease. Nevertheless, one third of patients in stage III and IV had MetS.
Stanciu et al. also investigated the prevalence of MetS in patients with COPD and found that 48.1% of COPD patients had MetS (25). Our result was also consistent with the result of Stanciu's and colleges' study. Marquis et al. reported high prevalence (61%) of MetS in men with COPD participating to a cardiopulmonary rehabilitation program (12). This high prevalence may be related to proportion of population included in the study that contained patients who had cardiovascular diseases. Hence, they might have had high probability of having MetS.
The prevalence of MetS was reported as 17.9% in a large population-based study in Turkey (26). Gemalmaz et al. found the prevalence as 38.1% in the study including smaller Turkish population (27). Gundogan et al. showed the prevalence as 34.6% in a Turkish population from Mediterranean region (28). The prevalence of MetS in COPD patient group in our study was higher than general Turkish population (44.6%). Besides, the prevalence in control group was consistent with the result of Sanisoglu et al., but lower than the other two studies (26,27,28).
When the parameters of MetS were evaluated one by one, hypertension had the highest frequency in COPD patients (77.2%). Similarly, Watz et al. showed that hypertension was highly prevalent in COPD patients (70%) (10). Whereas, Barr et al. found that frequency of hypertension in COPD patients was 55% (29). However, they used telephone questionnaire in contrast to objective measurement of blood pressure to determine presence of hypertension. This method may be responsible for the lower frequency of hypertension among COPD patients.
In our study, prevalence of abdominal obesity in COPD patients (52.2%) was the secondly frequent parameter of MetS just after hypertension. Visceral adipose tissue is an important source of IL-6 that induces production of high sensitivity CRP from hepatocytes (30). The study by Poulain et al. indicated that the presence of obesity, especially abdominal obesity, was associated with increased TNF-α and IL-6, and decreased adiponectin in plasma of patients with COPD (31). In our study, high sensitivity CRP levels were higher in COPD patients with MetS and 52.2% of COPD patients having abdominal obesity. Weight loss may be helpful to decrease the grade of systemic inflammation in COPD patients.
The third common parameter of MetS in COPD patients included in our study was hyperglycemia (46.7%). It was previously shown that there was increased prevalence of diabetes in COPD patients (4,32). Gudmundsson et al. suggested that mortality rate of patients with COPD and diabetes was increased during follow-up patients hospitalized because of exacerbation of COPD (33). It is not clear yet, whether systemic inflammation associated with COPD causes metabolic disorders or metabolic signals trigger inflammatory response (34).
Several markers of systemic inflammation such as hs-CRP, IL-6 were found higher in stable COPD patients than control subjects (2,35,36), suggesting low grade systemic inflammation even during clinical stability. CRP is one of the most widely used serum marker of systemic inflammation. Gl?ser et al. showed that higher levels of CRP were associated with decreased lung volumes in a general population over a wide range of age (37). In this study, high sensitivity CRP levels were also higher in stable COPD patients than control group. Moreover, CRP levels were higher in patients with MetS than the ones without MetS. This result may indicate that presence of MetS in COPD patients is associated with more intense systemic inflammation than it is in patients with COPD but without MetS. Similarly, Watz et al. also found that presence of MetS in patients with COPD was associated with significantly higher levels of hs-CRP (9). CRP levels were not significantly different between GOLD stages in our study. In contrast, Watz et al. showed that clinical stage of COPD was an independent factor predicting the levels of hs-CRP (9). Stanciu et al. reported that serum TNF-α and high sensitivity CRP levels were higher, whereas adiponectin levels were lower in patients with COPD and MetS than patients with COPD but without MetS (25). Our study also revealed that high sensitivity CRP levels were higher in patients with COPD and MetS than patients with COPD but without Mets, which was in accordance with the results of Stanciu and colleagues' study.
Smoking status of patient and control groups in this study was significantly different. This might be a determinant factor for difference in frequencies of MetS between two groups. However, considering their ages matched with COPD patients and normal spirometry, we selected control subjects randomly. Since smoking is the major risk factor for COPD, more intense smoking history in COPD group than control subjects is an expected result.
Our study has some limitations. First, the number of COPD patients included in the study was limited and patients were selected from only one center, to define exact prevalence of MetS in patients with COPD. Further studies that contain patients from many different centers are necessary. Secondly, a cross-sectional study was performed to determine the effect of presence of MetS to the course of COPD. Nevertheless, prospective studies may be more useful.
In conclusion, the results of this study showed that MetS, a determinant of systemic inflammation in general population, was more frequent in stable COPD patients, especially in early stages (GOLD stage I-II). The frequency of MetS in patient group was also higher than general Turkish population. The levels of high sensitivity CRP, as a marker of systemic inflammation, were higher in both patient and control groups with MetS than the ones without MetS. When the components of MetS were evaluated separately; abdominal obesity, hypertension and hyperglycemia were significantly more in patient group. Further studies are necessary to clarify existing mechanisms for the relationship between MetS and COPD.
REFERENCES
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) Updated 2008. Available from: http://www.goldcopd.org/
- Fabbri LM, Rabe K. From COPD to chronic systemic inflammatory syndrome? Lancet 2007; 370: 797-99.
- Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of co morbidities. Eur Respir J 2006; 28: 1245-57. [?zet] [Tam Metin] [PDF]
- Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J 2008; 32: 962-9. [?zet] [Tam Metin] [PDF]
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59: 574-80. [?zet] [PDF]
- Magnussen H, Watz H. Systemic inflammation in chronic obstructive pulmonary disease and asthma: relation with co morbidities. Proc Am Thorac Soc 2009; 6: 648-51. [?zet] [Tam Metin] [PDF]
-
Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al.
Cardiovascular morbidity and mortality associated with the metabolic syndrome.
Diabetes Care 2001; 24: 683-9.
[?zet] [Tam Metin] [PDF] - Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin-resistance syndrome (syndrome X). Diabetes 1992; 41: 715-22. [?zet]
- Watz H, Waschki B, Kirsten A, M?ler KC, Kretschmar G, Meyer T, et al. The metabolic syndrome in patients with chronic bronchitis and COPD. Chest 2009; 136: 1039-46. [?zet]
-
Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, et al. Airflow
obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur
Respir J 2010; 35: 317-23.
[?zet] [Tam Metin] [PDF] - Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord 2004; 2: 82-204. [?zet]
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, et al. Metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2005; 25: 226-32. [?zet]
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002; 287: 356-9. [?zet]
- Bolton CE, Evans M, Ionescu A, Edwards SM, Morris RH, Luzio S, et al. ?nsulin resistance and inflammation-a further systemic complication of COPD. COPD 2007; 4: 121-6. [?zet]
- Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometer. Circulation 1993; 88: 2460-70. [?zet]
- Lohmann T, Roche ARM. The Airlie (VA) consensus: standardization of anthropometric measurements. Human Kinetic Publishers, Champaign IL, 1988: 39-80.
-
Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. For the Conference
Participants. Definition of metabolic syndrome: report of the National Heart,
Lung, and Blood Institute/American Heart Association Conference on Scientific
Issues Related to Definition. Circulation 2004; 109: 433-8.
[?zet] [PDF] -
Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest 2011; 139: 165-73.
[?zet] - Barnes PJ, Celli BR. Systemic manifestations and co morbidities of COPD. Eur Respir J 2009; 33: 1165-85. [?zet] [Tam Metin] [PDF]
- Fabbri LM, Luppi F, Begh? B, Rabe KF. Complex chronic co morbidities of COPD. Eur Respir J 2008; 31: 204-12. [?zet] [Tam Metin] [PDF]
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 2004; 59: 574-80. [?zet] [PDF]
- Hurst JR, Vestbo J, Anzueto Locantore N, M?llerova H, Tal-Singer R, Miller B, et al. for the ECLIPSE Investigators: Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128-38. [?zet] [Tam Metin] [PDF]
- De Torres JP, Pinto-Plata V, Casanova C. C-reactive protein levels and survival in patients with moderate to very severe COPD. Chest 2008; 1333: 1336-43. [?zet]
- Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175: 250-5. [?zet] [Tam Metin] [PDF]
- Stanciu S, Marinescu R, Iordache M, Dumitrescu S, Mure?an M, Bo?dan MA. Are systemic inflammatory profiles different in patients with COPD and metabolic syndrome as compared to those with COPD alone? Rom J Intern Med 2009; 47: 381-6. [?zet]
-
Saniso?lu SY, Oktenli C, Hasimi A, Yokuso?lu M. Prevalence of metabolic
syndrome-related disorders in a large adult population in Turkey. BMC Public
Health 2006; 6: 92. doi:10.1186/1471-2458-6-92.
[?zet] [Tam Metin] [PDF] - Gemalmaz A, Ayd?n S, Ba?ak O, Dis?igil G, Korul A. Prevalence of the metabolic syndrome in a rural Turkish population: comparison and concordance of two diagnostic criteria. Turk J Med Sci 2008; 38: 159-65. [?zet] [PDF]
- G?ndo?an K, Bayram F, Capak M, Tanr?verdi F, Karaman A, Ozturk A, et al. Prevalence of metabolic syndrome in the Mediterranean region of Turkey: evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metab Syndr Relat Disord 2009; 7: 427-34. [?zet]
- Barr RG, Celli BR, Mannino DM. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med 2009; 122: 348-55. [?zet] [Tam Metin] [PDF]
-
Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin
Immunol 2005; 115: 911-9.
[?zet] - Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, et al. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chronic Respiratory Disease 2008; 5: 34-41. [?zet]
- Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2008; 31: 741-6. [?zet] [Tam Metin] [PDF]
-
Gudmundsson G, Gislason T, Lindberg E, Hallin R, Ulrik CS, Brondum E.
Mortality in COPD patients discharged from hospital: the role of treatment and
co-morbidity. Respir Res 2006; 7: 109-16.
[?zet] [Tam Metin] [PDF] - Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860-7. [?zet]
-
Karada? F, Kirdar S, Karul AB, Ceylan E. The value of C-reactive protein as
a marker of systemic inflammation in stable chronic obstructive pulmonary
disease. Eur J Intern Med 2008; 19: 104-8.
[?zet] - Broekhuizen R, Wouters EFM, Creutzberg EC, Schols AMWJ. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006; 61: 17-22. [?zet] [Tam Metin] [PDF]
- Gl?ser S, Ittermann T, Koch B, V?lzke H, Wallaschofski H, Nauck M, et al. Airflow limitation, lung volumes and systemic inflammation in a general population. Eur Respir J 2012; 39: 29-37. [?zet]
Yaz??ma Adresi (Address for Correspondence):
Dr. Evrim Eylem AKPINAR,
Ufuk ?niversitesi T?p Fak?ltesi,
G???s Hastal?klar? Anabilim Dal?,
Konya Yolu, No: 88/86
Balgat, ANKARA - TURKEY
e-mail: drevrimeylem@gmail.com