Uzat?lm?? inisial faz sonunda, smear pozitifli?inin devam etti?i t?berk?loz olgular?n?n sonucu
Aylin
BABALIK1, Haluk ?ALI?IR1, Nadi BAKIRCI2, H?lya
ARDA3, ?ule KIZILTA?1, Korkmaz ORU?1,
G?lg?n
?ET?NTA?1
1 SB S?reyyapa?a G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
G???s Hastal?klar? Klini?i, ?stanbul,
2 Ac?badem ?niversitesi T?p Fak?ltesi, Halk Sa?l??? Anabilim Dal?, ?stanbul,
3 SB S?reyyapa?a G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
Halk Sa?l??? B?l?m?, ?stanbul.
?ZET
Uzat?lm?? inisial faz sonunda, smear pozitifli?inin devam etti?i t?berk?loz olgular?n?n sonucu
Giri?: Bu ?al??mada kategori 1 tedavi rejiminde, ???nc? ay sonunda balgam pozitifli?i olan t?berk?loz hastalar?n?n tedavi sonucunun sunulmas? ama?land?.
Hastalar ve Metod: S?reyyapa?a G???s Hastal?klar? ve G???s Cerrahisi E?itim ve Ara?t?rma Hastanesinde Ocak 2004-Aral?k 2005 tarihleri aras?nda tedavi edilen 1024 t?berk?loz hastas? bu retrospektif kohort ?al??mas?na dahil edilmi?tir. T?berk?loz hastalar?n?n kategorizasyonu ve uygun tedavisi D?nya Sa?l?k ?r??t? rehberine uygun olarak yap?lm??t?r.
Bulgular: Alt? y?z elli be? (%64) kategori 1 tedavi alan hastadan (toplam hasta say?s? 1024) 11 (%2)'inin ???nc? ayda balgam smear pozitifli?inin devam etti?i saptand?. Devam faz? 11 hastada ba?land?. Balgam smear konversiyonu 10 hastadan alt?s?nda d?rd?nc? ayda, ?? hastada be?inci ayda, bir hastada alt?nc? ayda saptand?. ???nc? ay sonras? k?lt?r pozitifli?i devam etmedi. On bir olgunun 10'unda alt? ay maj?r ila?larla tedavi tamamland?. Be? y?l sonra hastalar de?erlendirildi ve hi? relaps olmad??? saptand?.
Sonu?: ?nisial faz sonunda balgam pozitifli?i devam eden hastalarda devam faz?na ge?ilmesi gerekir.
Anahtar Kelimeler: T?berk?loz, kategori 1 tedavi rejimi, uzam?? smear pozitifli?i, geni?lemi? inisial faz.
SUMMARY
The outcome of tuberculosis cases with persistent smear positivity at the end of extended initial phase
Aylin
BABALIK1, Haluk ?ALI?IR1, Nadi BAKIRCI2, H?lya
ARDA3, ?ule KIZILTA?1, Korkmaz ORU?1,
G?lg?n
?ET?NTA?1
1 Clinic of Chest Diseases, Sureyyapasa Chest Diseases and Chest Surgery Training and Research Hospital,
Istanbul, Turkey,
2 Department of Public Health, Faculty of Medicine, Acibadem University, Istanbul, Turkey,
3 Department of Public Health, Sureyyapasa Chest Diseases and Chest Surgery Training and
Research Hospital, Istanbul, Turkey.
Introduction: To present the treatment outcome in tuberculosis patients with sputum smear positivity in the third month of category 1 treatment regimes.
Patients and Methods: A total of 1024 patients with tuberculosis treated in Ministry of Health Sureyyapasa Chest Diseases and Chest Surgery Training and Research Hospital from January 2004 to December 2005 were included in this retrospective cohort study. Categorization and appropriate treatment of tuberculosis was performed according the World Health Organization guidelines.
Results: Of overall 1024 patients, 655 (64%) were determined to receive category 1 treatment while sputum smear positivity was identified in 11 of them [2%; mean (SD) age: 46 (17.9) years] in the third month. Continuation phase treatment was initiated in these 11 patients. Sputum conversion was evident in six of 10 cases in the 4th month, in three cases in the 5th month and in one case in the 6th month. None had culture positivity after the 3rd month. Of 11 cases, 10 completed therapy with major drugs in six months and treatment outcome was cure. No relapse was identified after five years later.
Conclusion: Based on our data we recommend that the continuing phase should be started in cases with positive sputum smear at the end of the extended initial phase.
Key Words: Tuberculosis, category 1 treatment regime, persistent smear positivity, extended initial phase.
Geli? Tarihi/Received: 08/10/2011 - Kabul Edili? Tarihi/Accepted: 13/08/2012
INTRODUCTION
Tuberculosis (TB) is chronic pulmonary disease associated with high morbidity and mortality rates. The estimates of the global burden of TB in 2009 indicated 9.4 million incident cases (1). According to the latest data from the Ministry of Health, 18.452 cases have been diagnosed with tuberculosis in Turkey in 2008 while new cases composed 90.8% (16.760 patients) of the patient population (2). Provided that microscopy laboratory services are available and diagnostic criteria are properly applied in a systematic way, pulmonary TB smear-positive cases represent at least 65% of the total pulmonary TB in adults and 50% or more of all TB cases (3).
Short-course regimens have been considered to achieve smear and culture conversion within 2-3 months in most patients. Many regimes achieve a favourable response, as defined by culture negativity at the end of the treatment in 97-100% of the treated population whereas the identification of practical regimens with low (< 5%) relapse rates has been the main therapeutic challenge (4). Sputum smear conversion after two or three months of treatment is a good predictor of eventual cure if treatment is completed. Smears should be converted to negative in the majority of new smear-positive pulmonary TB patients after two or three months of the anti-TB treatment (3).
A positive sputum smear at the end of the intensive phase may indicate poor supervision of the initial phase of therapy, poor patient adherence, poor quality of anti-TB drugs, use of anti-TB drugs at doses below the recommended range, slow resolution due to extensive cavitation and a heavy initial bacillary load, presence of co-morbid conditions that interfere either with adherence or with response, presence of drug-resistant Mycobacterium tuberculosis that is not responding to first-line treatment and the evidence of non-viable bacteria remain visible by microscopic examination (5).
There are different management strategies applied in patients who had smear positivity at the end of the initial phase. This study was designed to present the treatment outcome in our patients who had smear positivity end of the extended initial phase after which treatment was continued with two drugs according to WHO guidleness 2003.
PATIENTS and METHODS
A total of 1024 patients with TB treated in Ministry of Health Sureyyapasa Chest Diseases and Chest Surgery Training and Research Hospital from January 2004 to December 2005 were included in this retrospective cohort study.
TB Treatment
Categorization and appropriate treatment of TB was performed according the World Health Organization (WHO) guidelines (5,6). Category 1 was defined to be composed of TB patients with new smear-positivity, new smear-negative pulmonary TB with extensive parenchymal involvement and severe forms of extra-pulmonary TB. Category 2 was defined to be composed of TB patients with previously treated sputum smear-positive pulmonary TB including relapse, treatment after interruption and treatment failure. Category 3 was defined to be composed of TB patients with new smear-negative pulmonary TB (other than in category 1) and less severe forms of extra-pulmonary TB. Category 4 was defined to be composed of TB patients with chronic and multi-drug resistant TB cases (still sputum-positive after supervised re-treatment).
Treatment regime administered in category 1 composed of two phases including initial phase of two months with isoniasid (H), rifampicine (R), pyrazinamide (Z) and ethambutol (E) therapy under direct observation followed by continuation phase including four months self-administration of HR therapy. Category 2 treatment regime composed of initial phase of two months "HRZE and streptomycine (S)" followed by one month HRZE under direct observation and continuation phase of five months self-administration of HRE therapy. Category 4 treatment regime included standardized treatment regime with second-line drugs (7).
In case of failure of therapy in category 1 patients, category 2 treatment was initiated while the treatment failure in category 2 patients was managed by administration of category 4 treatment regime.
Study Procedures
In case of identification of positive sputum examination at the end of the initial phase one month extension was performed during the treatment of category 1 patients. Then, at the end of this additional month, if the smear sputum was still positive, the continuing phase was started with two major drugs (HR) under supervision at monthly check-ups. Patients were hospitalized until the sputum conversion was achieved. After discharge, sputum samples of patients were checked at the second month, fifth month and at the end of the treatment regime.
Bacteriological testing was based on three sputum samples per patient or a gastric aspiration sample if the patient could not produce sputum. The sputum and gastric aspiration samples were evaluated for acid-fast bacillus (AFB) by Erlich Ziehl Neelsen (EZN). Sputum smear examination was performed at least once in a month during treatment until achievement of sputum conversion and following discharge of the patient.
Sputum culture was performed on a monthly basis via L?wenstein-Jensen medium for M. tuberculosis. Routine drug sensivity tests were performed for H, R, E, S at the beginning of the treatment in all patients identified with culture positivity. Drug susceptibility tests (DST) were performed by proportion method but without an external laboratory quality control system. DST was not used to lead the management of the patients.
Assessment of Treatment Outcome
Treatment outcome was assessed and sputum conversion time was recorded yearly. Treatment outcome was defined according to WHO guidelines including cure, treatment completion, treatment failure, death, default and transfer out classifications. "Cure" was considered if a patient's sputum smear or culture was positive at the beginning of the treatment but negative in the last month of the treatment and on at least one previous occasion. "Treatment completion" was considered if a patient completed the treatment but failed to have a negative sputum smear or culture in the last month of treatment and on at least one previous occasion. "Treatment failure" was considered if a patient whose sputum smears or culture was positive at five months or later during treatment. Also included in this definition were patients who were determined to harbor a multi-drug resistant strain at any point of time during the treatment, whether they were smear-negative or -positive. "Death" was considered as the treatment outcome if a patient dies for any reason during the course of treatment. "Default" was considered if treatment was interrupted for two consecutive months or more. "Transfer out" was considered if a patient was transferred to another recording and reporting unit and whose treatment outcome was unknown.
Category 1 TB patients who had sputum smear-positive at the end of extended initial phase were evaluated retrospectively based on medical records in terms of age, drug resistance, bilateral or unilateral and cavitary radiological lesions, co-morbidities, drug related side effects and the treatment outcomes. Relapse occurrence in these patients was evaluated by direct phone calling of patients or TB dispensary five years later.
RESULTS
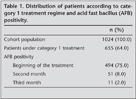
Of 1024 patients, 655 (64%) patients received category 1 treatment regimes; 494 (75%) of whom were sputum smear positive at the beginning of treatment. Sputum smear positivity was identified in 51 (8%) patients the second month, and in 11 (2%) the third month (Table 1). All 11 patients with sputum smear positivity at the third month of category 1 treatment regimes were males with mean (SD) age of 46 (17.9) years.
Continuation phase treatment was initiated in these 11 patients. Treatment was altered with second-line drugs after the 4th month only in one patient while remaining 10 cases completed therapy with major drugs in six months.
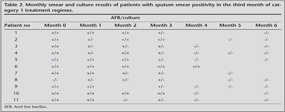
Sputum conversion was evident in 6 of 10 cases in the 4th month, in three cases in the 5th month and in one case in the 6th month. In nine of 10 cases last sputum check was performed in the 6th month, while in one case in the 5th month. Considering monthly culture results, seven of 11 cases had culture positivity in the 1st month, six cases in the 2nd month, three cases in the 3rd month. None had culture positivity after the 3rd month (Table 2).
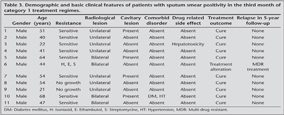
DST was performed in nine of 11 cases; and in eight? cases, sensitivity for all drugs was detected. One case had drug resistance for isoniasid, rifampicin, and streptomycin. Bilateral radiologic lesions were determined in four of 11 cases and two of 11 cases had cavitary lesions.
Due to bilateral lesion, drug resistance and worsening clinic situation during the treatment, one case's treatment was changed with second-line drugs in the 4th month. Standardized treatment regime was completed with second-line drugs 18 months after culture conversion and treatment outcome was cure with second-line drugs (Table 3).
Of 11 cases, 10 cases completed therapy in six months and treatment outcome was cure in these patients. For all 11 cases, after the five years, follow-up was done by direct phone calling of patients or TB dispensary for evaluating relapse. No relapse was identified after five years later (Table 3).
Diabetes and hypertension were identified co-morbid diseases in one of 11 cases (Table 3).
Hepatotoxicity was the drug related side effects identified in one patient based on symptoms of nausea and vomiting and the elevated liver function tests occurred on the 30th day of the treatment. Treatment was withdrawn for 13 days in this patient until normalization of liver function tests after which all drugs were administered in normal doses and treatment was completed in six months.
DISCUSSION
Six months of daily treatment with rifampicin and isoniazid, supplemented in the initial two months with pyrazinamide and either ethambutol or streptomycin (the six-month four-drug regimen) has been the evidence-based gold standard for TB treatment for at least the last 15 years. No new first-line drugs have been introduced for over 30 years (5,8).
Examination of smear-positive patients in two months, five months, and at the end of treatment provide a better indication of the success of treatment in large-scale treatment programmes (9). The proportion of smear-positive patients with sputum smear conversion at the end of the intensive phase has also been considered as an indicator of TB programme performance (5). However, at the end of the second month of therapy, skilled laboratory technicians can often detect low grades of positivity, while the positivity rate can still be as high as 25%, even if the initial phase of treatment is well supervised and the drugs are of good quality (3).
Horne et al. performed a systemic review and meta-analysis to evaluate the accuracy of a positive sputum smear or culture during treatment with standardized regimen with rifampin in the initial phase for predicting failure or relapse in pulmonary TB. As a result, both culture and smear had low PPV (positive predictive value) ranging from nine to 18% in predicting poor outcome, suggesting a low probability that a positive sputum specimen at any month could correctly predict the failure or relapse. In contrast, NPV (negative predictive value) were high (at least 93%), indicating a negative sputum test result at any month of treatment makes relapse or failure unlikely (10).
According to ATS recommended regimes, the continuation phase should be extended for an additional three months for patients who have cavitation on initial or follow up chest radiography and are culture-positive at the time of completion of initial phase of treatment (two months) (11).
Previous WHO Guideline recommends that whatever the reason, if the sputum is positive at the end of the second month, the initial phase is prolonged for a third month. Smears may be checked at the end of third month to evaluate smear conversion in the cohort (6). But according to the latest WHO guideline, in patients treated with the regimen containing rifampicin throughout treatment, if a positive sputum smear is found at completion of the intensive phase, the extension of the intensive phase is not recommended. In new patients, if the specimen obtained at the end of month three is smear-positive, sputum culture and DST should be performed (5).
Turkish Ministry of Health published a recommendation book for tuberculosis care which guides many physicians in therapeutic decisions. New smear positive pulmonary TB are treated with a six month short-course chemotherapy (category 1) regimen according to this book which recommends to continue with the same four drugs at the initial phase (12).
For 11 cases that had smear positivity at the end of the extended initial phase, continuations phase was begun with two drugs. Treatment outcome of 10 cases was cure indicating high cure rate in these patients (91%) in our study population. Only one patient who had poor clinical status and drug resistance as well as bilateral radiological lesions was treated with second-line drugs after the 4th month (9%).
Risk factors such as higher age, higher smear grading, presence of cavitary disease, extensive disease, co-morbid conditions like diabetes mellitus have been considered to be associated with a delay in smear conversion among TB patients (13,14,15,16,17). Rekha et al. showed that at the end of initial phase, the smear and culture conversion rates were similar in diabetes mellitus and HIV groups (18).
Certain risk factors were also valid in our patients including higher age (nine of 11 cases were older than 40 years), presence of bilateral radiological lesions (four of 11 cases), cavitary lesions (three of 11 cases) and co-morbid disorders (one of 11 cases). Drug related side effect was observed in one patient but after the 13 day treatment interruption, normal full therapeutic doses were re-administrated.
Smear status at the end of the intensive phase is a poor predictor of relapse development. Frequency of relapse rate with standardized short-course chemotherapy was reported to be around 3-7%. Approximately 80% of the relapses have been considered to occur within the first six months of treatment withdrawal while after 3-5 years, the risk in both groups diminishes appreciably (19).
Some studies showed that relapse rate was high for who had inadequate duration of treatment and irregular treatment. In our study, evaluation of patients with smear positivity at the end of the extended phase after five years later revealed that neither 10 cases who had treated short course treatment nor one case who treated with second-line drugs relapsed.
The clinical management of patients with smear positivity at the end of the initial phase varies considerably. Several physicians prefer not to pass to the continuation phase if patient's sputum is positive for AFB at the end of the extended period. Others prefer to continue therapy with the same drugs at the initial phase of the treatment extending total duration of treatment.
Encounterence of sputum smear positivity in the third month of anti-TB therapy is known to be quite seldom in the clinical practice. Accordingly, only 2% of our patients receiving category 1 treatment regimes were identified to have sputum smear positivity in the third month.
In our opinion, continuation with the same treatment after the extended initial phase treatment with four major drugs may lead to neglect of standard regime as well as evaluation of tuberculosis patients in accordance with the standard protocol. In this sense, administration of standard short course TB treatment according to WHO guidelines may enhance the management of the disease in the primary health care system. Use of hepatotoxic drugs for long time may lead to adverse drug reaction while use of multiple drugs for a long time lead to decrease treatment compliance.
In conclusion, based on our data we recommend that the continuing phase should be started in cases where patients have sputum smear positive outcomes at the end of the extended initial phase.
CONFLICT of INTEREST
None declared.
REFERENCES
- Global Tuberculosis Control. WHO Report 2010. WHO/HTM/TB/2010.7
- Republic of Turkey Ministry of Health, Department of tuberculous Struggle, Tuberculosis in Turkey, 2010.
- COMPEDIUM of indicators for monitoring and evaluating national tuberculosis programs WHO/HTM/TBT 2004.344
- Santha T. What is the optimum duration of treatment? In: TOMAN'S tuberculosis cases detection, treatment, and monitoring. WHO Geneva. 2nd ed. 2004: 144.
- Treatment of tuberculosis guidelines. 4th ed. World Health Organization. WHO/HTM/TB/2009.420
- Treatment of Tuberculosis: Guidelines for national programmes. World Health Organization-Geneva. 3rd ed. 2003.
- Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB/2006.361
- National Collaborating Centre for Chronic Conditions and the Centre for Clinical Practice at NICE. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. NICE clinical guideline 117, March 2011.
- Santha T. How can the progress of treatment be monitored ? In: TOMAN'S tuberculosis cases detection, treatment, and monitoring WHO Geneva. 2nd ed. 2004: 250.
- Horne DJ, Royce SE, Gooze L, Narita M, Hopewell PC, Nahid P, et al. Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. Lancet Infect Dis 2010; 10: 387-94.
- American Thoracic Society Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of Tuberculosis. Am J Respir Crit Care Med 2003; 167: 603-62.
- Republic of Turkey Ministry of Health, Department of Tuberculous Struggle. Reference Book for the Control of Tuberculosis in Turkey, 2003. www.verem.org.tr
- Singla R, Osman MM, Khan N, Al-Sharif N, Al-Sayegh MO, Shaikh MA. Factors predicting persistent sputum smear positivity among pulmonary tuberculosis patients 2 months after treatment. Int J Tuberc Lung Dis 2003; 7: 58-64.
- Wang JY, Lee LN, Yu CJ, Chien YJ, Yang PC; Tami Group. Factors influencing time to smear conversion in patients with smear-positive pulmonary tuberculosis. Respirology 2009; 14: 1012-9.
- G?ler M, Unsal E, Dursun B, Aydin O, Capan N. Factors influencing sputum smear and culture conversion time among patients with new case pulmonary tuberculosis. Int J Clin Pract 2007; 61: 231-5.
- Telzak EE, Fazal BA, Pollard CL, Turett GS, Justman JE, Blum S. Factors influencing time to sputum conversion among patients with smear-positive pulmonary tuberculosis. Clin Infect Dis 1997; 25: 666-70.
- Dom?nguez-Castellano A, Muniain MA, Rodriguez-Bano J, Garcia M, Rios MJ, Galvez J, et al. Factors associated with time to sputum smear conversion in active pulmonary tuberculosis. Int J Tuberc Lung Dis 2003; 7: 432-8.
- Banu Rekha VV, Balasubramanian R, Swaminathan S, Ramachandran R, Rahman F, Sundaram V, et al. Sputum conversion at the end of intensive phase of Category-1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection: an analysis of risk factors. Indian J Med Res 2007; 126: 452-8.
- Santha T. How important is follow-up and what is the frequency of relapse after the completion of treatment? In: Frieden T (ed). 2nd ed. TOMAN'S Tuberculosis Cases Detection, Treatment, and Monitoring. WHO Geneva: 2004: 267-9.
Yaz??ma Adresi (Address for Correspondence):
Dr. Aylin BABALIK,
SB S?reyyapa?a G???s Hastal?klar? ve
G???s Cerrahisi E?itim ve Ara?t?rma Hastanesi,
G???s Hastal?klar? Klini?i,
?STANBUL - TURKEY
e-mail: aylinbabalik@gmail.com